Basic Biochemistry
Atoms and Ions
-
All matter is composed of atoms
-
Atoms are made up of protons, neutrons and electrons
-
Atoms are most stable when their electron shells are complete A
-
toms can lose or gain electrons to achieve this state – they become charged ions
-
The chemical properties of an element are determined by the number of electrons in the atom
-
Atoms gaining electrons are reduced to negative ions
-
Atoms losing electrons are oxidised to positive ions
Bonding
-
Covalent bonds
-
electrons are shared between two atoms completing both outer shells
-
a molecule is formed - eg CO2
-

-
Ionic bonds
-
electrons are given from one atom to another
-
the resulting + and – charges form an electrostatic bond Eg NaCl
-
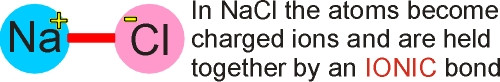
-
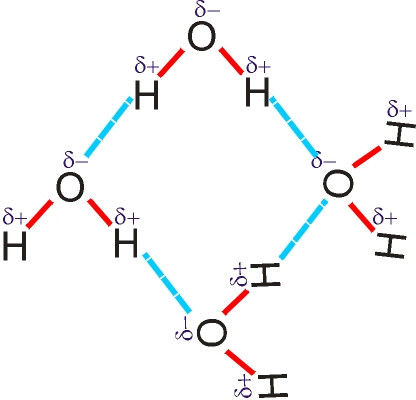
Hydrogen bonds
-
electrons are not evenly shared
-
one atom becomes slightly –ve and the other slightly +ve forming a weak electrostatic bond.
-
Many of these can have a significant bonding effect. Eg between water molecules
-