Biochemical tests
1. Tests for carbohydrates in the laboratory
Benedict’s test used to identify reducing sugars (monosaccharides and some disaccharides)
- Add Benedict’s solution to the chemical sample and heat.
- The solution changes from blue to brick-red or yellow if a reducing sugar is present.
Non-reducing sugar test used to test for non-reducing sugars, e.g. the disaccharide, sucrose
- First a Benedict’s test is performed.
- If the Benedict’s test is negative, the sample is hydrolysed by heating with hydrochloric acid, then neutralised with sodium hydrogen carbonate.
- This breaks the glycosidic bond of the disaccharide, releasing the monomers.
- A second Benedict’s test is performed which will be positive because the monomers are now free.
Starch test
- Add iodine solution to the sample.
- If starch is present the colour changes to blue-black.
All the biochemical tests need to be learned. This work is good value because they are regularly tested in 2 or 3 mark question components.
2. Tests for lipids in the laboratory
Emulsion test used to identify fats and oils
- Add ethanol to the sample, shake, then pour the mixture into water.
- If fats or oils are present then a white emulsion appears at the surface.
3. Tests for proteins in the laboratory
Biuret test used to identify any protein
- Add dilute sodium hydroxide and dilute copper sulphate to the sample.
- A violet colour appears if a protein is present.
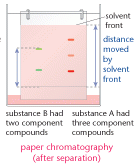
Chromatography
This technique is used to separate out the components in a mixture. It is used to separate out the components of substances such as chlorophyll, and can be used to help identify substances. The method is outlined below:
- a spot of the substance is placed on chromatography paper and left to dry
- the paper is suspended in a solvent such as propanone
- as the solvent molecules move through the paper the components begin to move up the paper, big molecules move slower than small ones. You need to remember that this technique separates substances in terms of the relative size of the molecules.
- the small solvent molecules move through the paper faster than any of the components of the substance
- the substance separates out into different spots or bands.

Rf value of a substance
This is calculated after the distances moved by compounds and solvent up the chromatogram have
been measured. The distance moved by the solvent is called the solvent front.
Rf value = distance moved by substance
distance moved by solvent front
Different compounds show up as different coloured bands. The technique shows the number of
compounds in the mixture. When this method is used on chlorophyll, five colours separate out.
The longer the chromatogram is left after the start, the higher the spots or bands ascend. For this
reason every compound has its Rf value calculated. However long the chromatogram is left, the Rf value is the same when using the same solvent.
