Carbohydrates
Structure of Carbohydrates
Monosaccharides
All carbohydrates are formed from the elements carbon (C), hydrogen (H) and oxygen (O). The formula of a carbohydrate is always (CH2O)n. The n represents the number of times the basic CH2O unit is repeated, e.g. where n = 6 the molecular formula is C6H12O6. This is the formula shared by glucose and other simple sugars like fructose. These simple sugars are known as monosaccharides.
The molecular formula, C6H12O6, does not indicate how the atoms bond together. Bonded to the carbon atoms are a number of – H and – OH groups. Different positions of these groups on the carbon chain are responsible for different properties of the molecules. The structural formulae of α and β glucose are shown below.
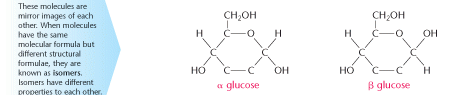
Glucose is so small that it can pass through the villi and capillaries into our bloodstream. The molecules subsequently release energy as a result of respiration. Simple glucose molecules are capable of so much more. They can combine with others to form bigger molecules.
Disaccharides
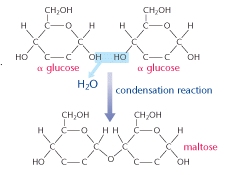
Each glucose unit is known as a monomer and is capable of linking others. This diagram shows two molecules of β glucose forming a disaccharide.
In your examinations look for different monosaccharides being given, like fructose or α glucose. You may be asked to show how they bond together. The principle will be exactly the same.
A condensation reaction means that as two carbohydrate molecules bond together a water molecule is produced. The link formed between the two glucose molecules is known as a glycosidic bond.
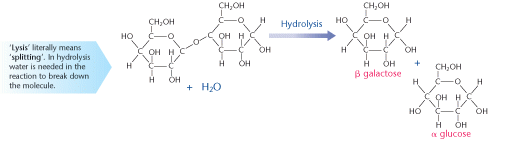
A glycosidic bond can also be broken down to release separate monomer units. This is the opposite of the reaction shown above. Instead of water being given off, a water molecule is needed to break each glycosidic bond. This is called hydrolysis because water is needed to split up the bigger molecule.
Polysaccharides
Like disaccharides, they consist of monomer units linked by the glycosidic bond. However, instead of just two monomer units they can have many. Chains of these ‘sugar’ units are known as polymers. These larger molecules have important structural and storage roles.
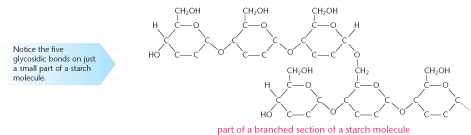
Starch is a polymer of the sugar, glucose. The diagram below shows part of a starch molecule.
The table classifies carbohydrates

How useful are polysaccharides?
- Starch is stored in organisms as a future energy source, e.g. potato has a high starch content to supply energy for the buds to grow at a later stage.
- Glycogen is stored in the liver, which releases glucose for energy in times of low blood sugar.
Both starch and glycogen are insoluble which enables them to remain inside cells.
- Cellulose has long chains and branches which help form a tough protective layer around plant cells, the cell wall.
- Pectins are used alongside cellulose in the cell wall. They are polysaccharides which are bound together by calcium pectate. Pectins help cells to bind together.
Together the cellulose and pectins give exceptional mechanical strength. The cell wall is also permeable to a wide range of substances.
