Phenols
After studying this section you should be able to:
- state the uses of phenols in antiseptics and disinfectants
- describe the reactions of phenol with sodium and bases
- explain the ease of bromination of phenol compared with benzene
Phenols
General structure: ArOH (Ar is an aromatic ring)
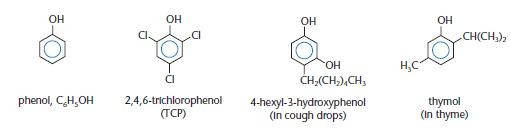
Phenols have an aromatic ring bonded directly to a hydroxyl (–OH) group. Some phenols are shown below.
Uses
Dilute solutions of phenol are used as disinfectants and phenol was probably the first antiseptic. However, phenol is toxic and can cause burns to the skin. Less toxic phenols are used nowadays in antiseptics. Phenols are also used in the production of plastics, such as the widely used phenolformaldehyde resins. More complex phenols, such as thymol shown above, can be used as flavourings and aromas and these are obtained from essential oils of plants.
Properties
Like alcohols, a phenol has a hydroxyl group which is able to form intermolecular hydrogen bonds. The resulting properties include:
- higher melting and boiling points than hydrocarbons with similar relative molecular masses
- some solubility in water.
Reactions of phenol as an acid
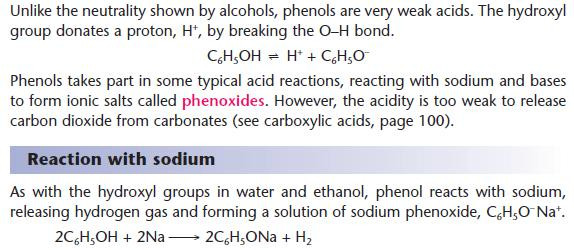
Key points from AS: Tests for alcohols

- Compare these reactions with those of carboxylic acids
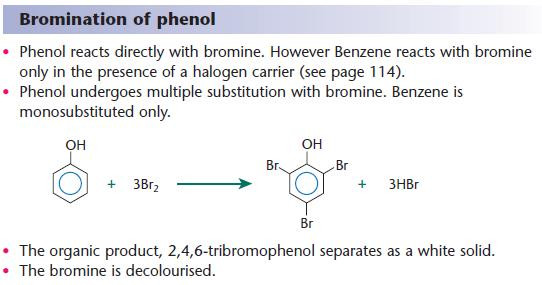
Electrophilic substitution of the aryl ring of phenol
The aryl ring of phenol has a greater electron density than benzene because the adjacent oxygen reinforces the electron density in the ring.
This results in:
- a greater electron density in the aryl ring
- greater reactivity of the ring towards electrophiles
Formation of esters
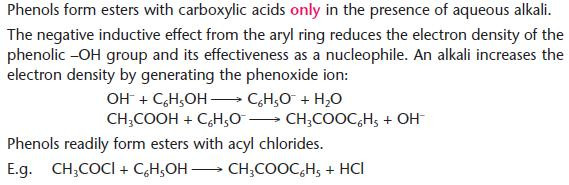
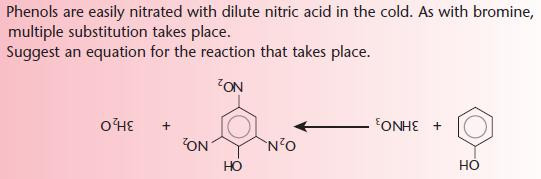
PROGRESS CHECK