Atoms and Isotopes
Atoms
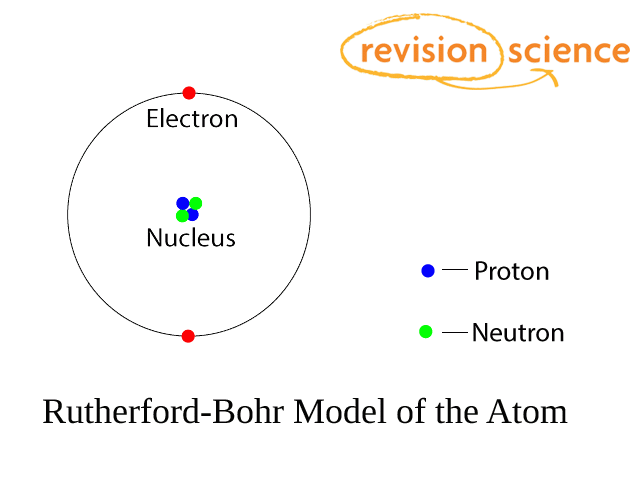
Atoms have a small central nucleus made up of protons and neutrons around which there are electrons.
Image
| PARTICLE | RELATIVE MASS | RELATIVE CHARGE |
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 1/1840 or 0.0005* | -1 |
*Because an electron has a negligible mass compared to protons and neutrons in most of Chemistry it can be ignored.
Isotopes
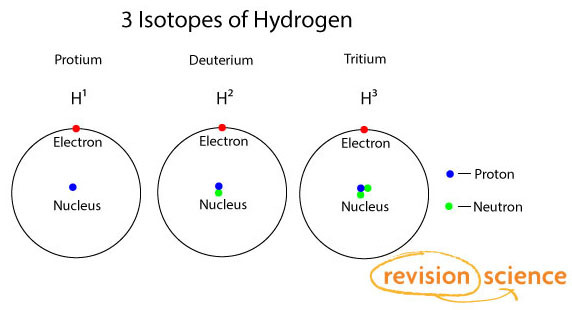
Atoms of the same element always have the same number of protons.
Isotopes are atoms of the same element but with different number of neutrons.
This gives rise to different mass numbers.
Relative abundance is the amount of each isotope as the percentage for that element occurring on the Earth
Image
| Isotope | Protons | Neutrons | Electrons | Atomic Number | Mass Number |
| 126C | 6 | 6 | 6 | 6 | 12 |
| 136C | 6 | 7 | 6 | 6 | 13 |
| 146C | 6 | 8 | 6 | 6 | 14 |
All Carbon isotopes have the same atomic number. The atomic number is sometimes omitted. i.e. 14C or carbon-14.
Isotopes of an element react the same way as chemical reactions depend on electrons, not neutrons.
