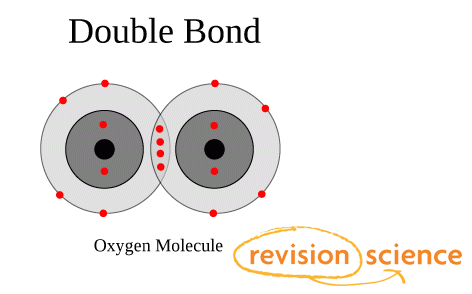
Bonded and Lone Pairs
Bonding pairs and lone pairs of electrons are charge clouds that repel each other.
A lone pair refers to a pair of valence electrons that are not shared with another atom and is sometimes called a non-bonding pair.
Image
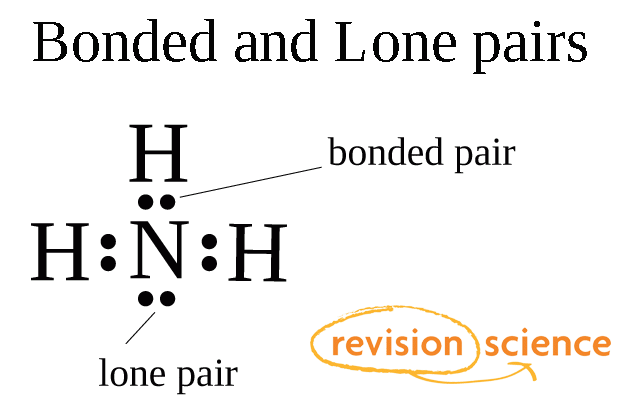
Lone pairs are found in the outermost electron shell of atoms.
Image
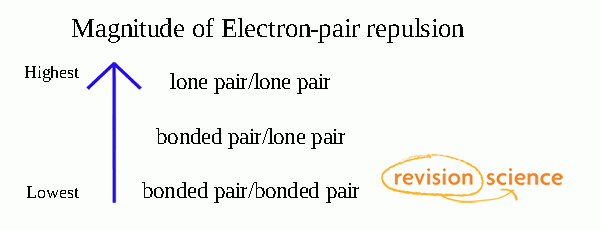
A double bond is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two.
Image