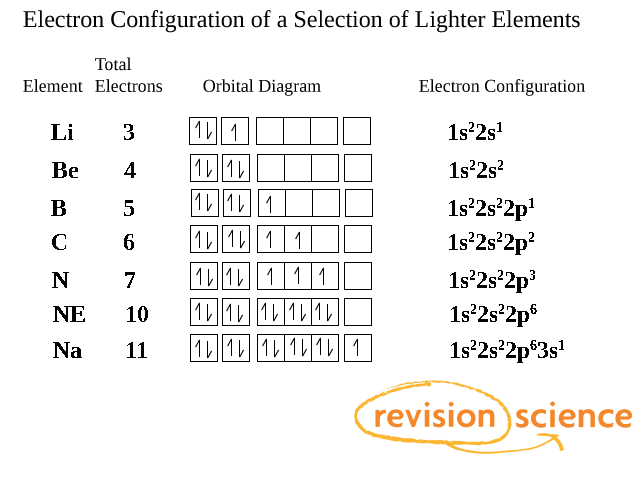
Electrons filling Subshells and Orbitals
Image
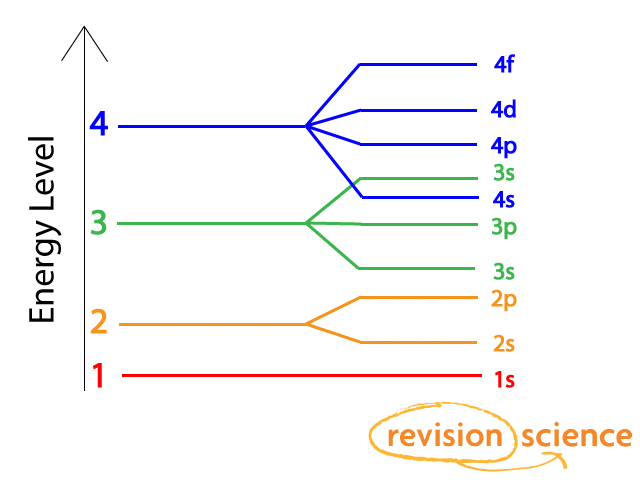
Subshells have different energy levels. In the diagram above you can see that the 3d energy level is actually higher than the 4s energy level.
Shells and subshells are filled in energy level order, so electrons will fill the 4s subshell before the 3d shell.
Orbitals are filled singly by electrons and will only double up once all orbitals have at least one electron to prevent repulsion by pairing.
Image