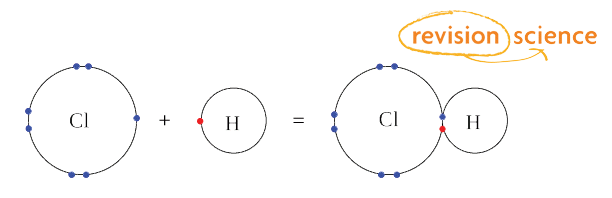
Octet Rule
Noble gases are very stable as their outermost shell is completely full, it has 8 electrons that are paired.
Atoms 'want' to be in this stable state; this is the 'Octet Rule'
When elements bond so that the resulting compound has a full outer shell of paired electrons they are said to obey the 'octet rule'.
There are exceptions to the Octet Rule:
-
Molecules with an odd number of electrons
-
Molecules in which an atom has less than an octet
-
Molecules in which an atom has more than an octet
Image