Orbitals
Subshells are made up of negative charge clouds called orbitals. Each orbital can hold up to 2 electrons.
Each subshell has a different type of orbital, an s subshell has an s-orbital, a p subshell p-orbitals, etc.
| Subshell | Orbitals | Electrons |
| s | 1 | 1 x 2 = 2 |
| p | 3 | 3 x 2 = 6 |
| d | 5 | 5 x 2 = 10 |
| f | 7 | 7 x 2 = 14 |
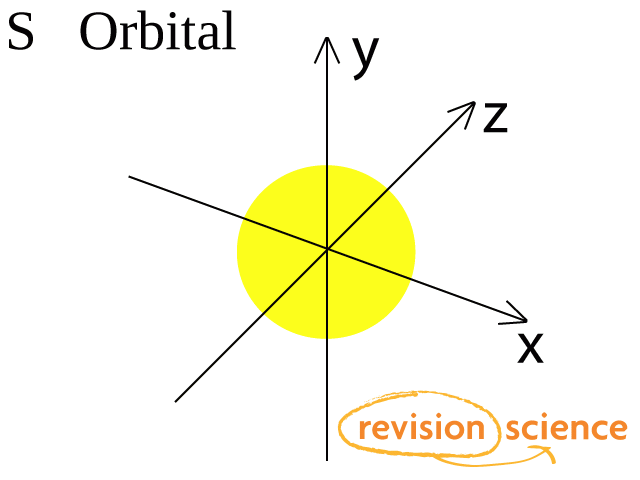
A s-orbital has a spherical shape.
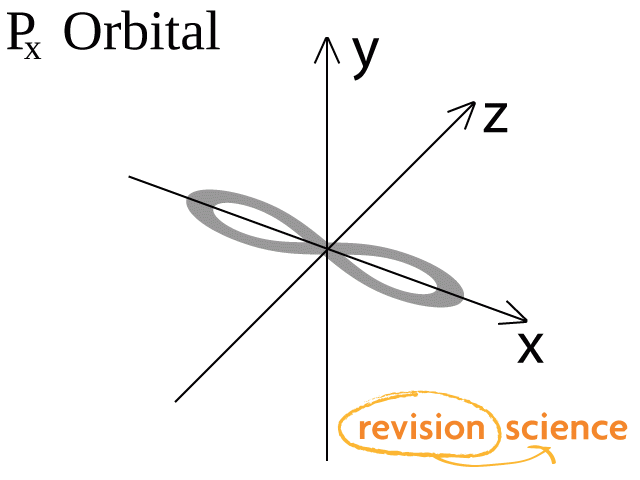
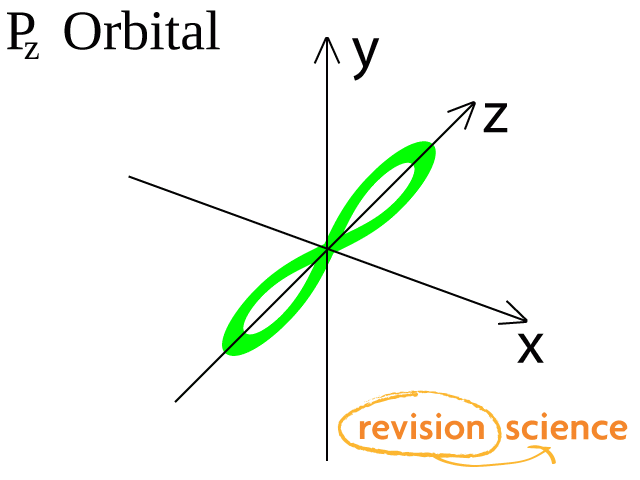
A p-orbital has a 3-dimensional dumb-bell shape.
There are three p-orbitals, px, py, and pz at right angles to one another.

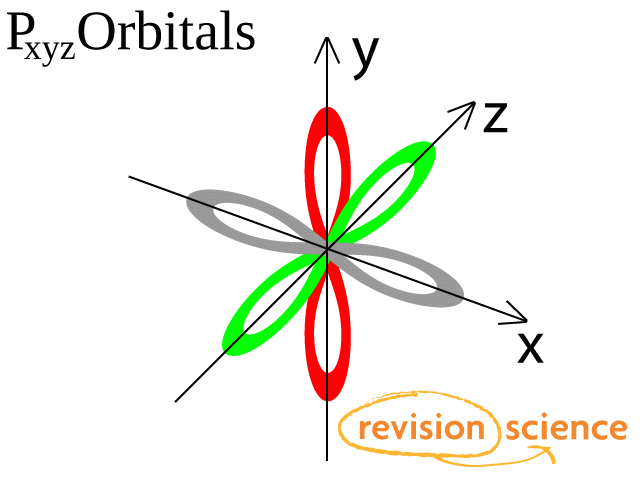
The structures of d and f-orbitals are more complex.
• There are five d-orbitals.
• There are seven f-orbitals.
Why do orbitals have 2 electrons?
Electrons have negative charge and will repel each other. However they have a property called spin.
The two electrons in an orbital will have different spins that helps overcome any repulsive energy.
Orbitals can be represented by a box with electrons represented by arrows within the box.
The spin of the electron is shown by having the arrow point either up or down.
Within an orbital the 2 arrows can't point in the same direction, the electrons must have opposite spin.
This video shows an introduction to Orbitals

