Empirical and Molecular Formula
Molecular formula show the actual number of atoms of the elements in a compound. The molecular formula for hydrogen peroxide is H2O2.
Empirical formula show the simplest, integer ratio of the atoms of the elements in a compound. The empirical formula for hydrogen peroxide is HO.
Different Molecular formula may result in the same Empirical formula.
|
Example |
Molecular Formula |
Empirical Formula |
|
Formaldehyde |
CH2O |
CH2O |
|
Acetic Acid |
C2H4O2 |
CH2O |
|
Glucose |
C6H12O6 |
CH2O |
Calculating Empirical Formula
If you are given the Percentage Composition of the Element:
It's best to work in grams. If values are given as a percentage assume the mass to be 100g and then the percentages become grams. (e.g 20% becomes 20g)
Work out the moles of each element. This is (sample mass)/(molar mass).
Work out the mole ratio by dividing each elements number of moles by the smallest value from the preceding step.
If the results are not whole numbers double or triple, etc. all values until you are left with only integers in the mole ratio.
If you are given a Combustion Analysis:
Calculate the number of grams of carbon in the compound by calculating the number of grams of carbon in the given amount of CO2.
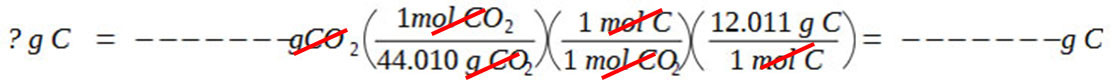
Calculate the number of grams of hydrogen in the compound by calculating the number of grams of hydrogen in the given amount of H2O.
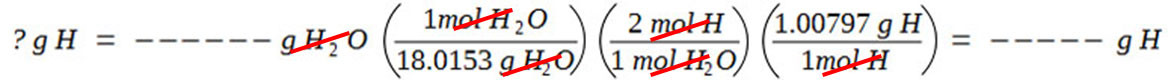
If the compound contains oxygen, calculate the number of grams of oxygen in it by subtracting the masses of carbon and hydrogen from the given total mass of compound.
? g O = (given) g total - (calculated) g C - (calculated) g H
Calculate the empirical formula of the compound from the grams of carbon, hydrogen, and oxygen.
Calculating Molecular Formula
(You are given the molecular mass for questions like this)
Work out the mass of the empirical formula.
Take the molecular mass and divide by the result from the previous step.
Multiply the atoms in the empirical formula by this result.
QUESTIONS
Calculate the empirical formula for each of the following substances.
You should use the following values for relative atomic mass:
H = 1 N = 14 O = 16 P = 31 S = 32 Cu = 64
1. What is the empirical formula of the phosphorus oxide that has 43.7% by mass of phosphorus and 28.4% by mass oxygen?
2. An oxide of nitrogen has 69.6% by mass of oxygen. What is the empirical formula?
3. What is the empirical formula of this compound of copper. It has the following percentages by mass. Cu 25.9% S 12.7% O 57.4% H 4%
Answers
1. P2O5
2. NO2
3. CuSO9H10
Formula from Mass Composition
Figuring out the empirical formula from a molecules mass composition
