Intermolecular Forces
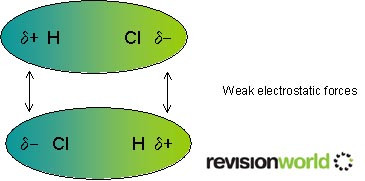
The easiest kind to understand are permanent dipole-permanent dipole interactions. These occur between polar molecules. A molecule is polar when there is an uneven distribution of electron density. This occurs in a bond when the atoms at each end have a different pull on the electron pair. The "amount of pull" is known as electronegativity. Fluorine is the most electronegative element (electronegativity value of 4) and the other elements have decreasing values as you go down the periodic table and as you go left.
The negative end of one molecule is attracted to the positive end of another.
Although pairs of molecules can form like in this illustration, chains can also be formed. The main point is that there is some attraction between the molecules and this makes them harder to separate and so have a higher boiling point than expected for non-polar molecules.
They are called permanent because the molecule always has this polarity.
This brings us to the next question, why do non-polar molecules stick together?
Consider two non-polar molecules colliding with each other. Remember that electrons are not held in fixed places but are distributed in clouds. Think of the molecules as being a plate of jelly!
As the molecules collide, their electron clouds are distorted and so, for a short time, the electrons are not evenly distributed around the molecules. The jellies have wobbled to one side. For that short time there is a temporary dipole within the molecules.
For that instant, opposite charges will attract and similar charges will repel. The attractive bits get closer together (more attraction), the repulsive bits get further apart (less repulsion). Overall, there is a small amount of attraction. As the electron clouds recover from their collision, they will bounce back to their original distribution but other collisions will repeat the process.
If the molecule is small, with fairly tightly held electrons (eg H2) , the electron distortion (jelly wobble) is small and so the attraction between those molecules is small. If the molecule is larger (eg I2), the electron distortion is larger. This explains why hydrogen is a gas even at very low temperatures yet iodine is a solid at room temperature.
The molecule is said to possess a temporary dipole, because it is only present for an instant (but it is continually reformed).
It is interesting to consider which is the most important of these effects.
Intuitively, most people would say that the permanent dipole dipole attraction is the most important. However, if you look at the boiling point of the hydrogen halides (HCl, HBr, HI) you will see that the boiling point increases as you get the bigger halogens (more temporary dipoles) even though the bond polarity is less (weaker permanent dipoles). This shows that the increase in the temporary dipole-dipole attraction is bigger than the decrease in the permanent dipole-dipole attraction.
