The Nature of Bonds
Covalent Bonding
The atoms are held together by one or more shared pairs of electrons. The electrons come from the highest energy level of each atom. One pair of electrons makes a single bond, two pairs make a double bond (such as in alkenes or carbon dioxide etc), three pairs make a triple bond (as in alkynes or in a nitrogen molecule)
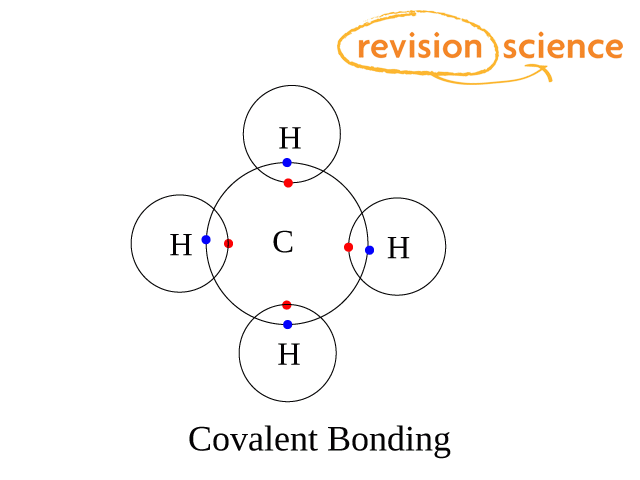
A co-ordinate (or datie covalent) bond is a covalent bond in which both bonding electrons come from the same element.
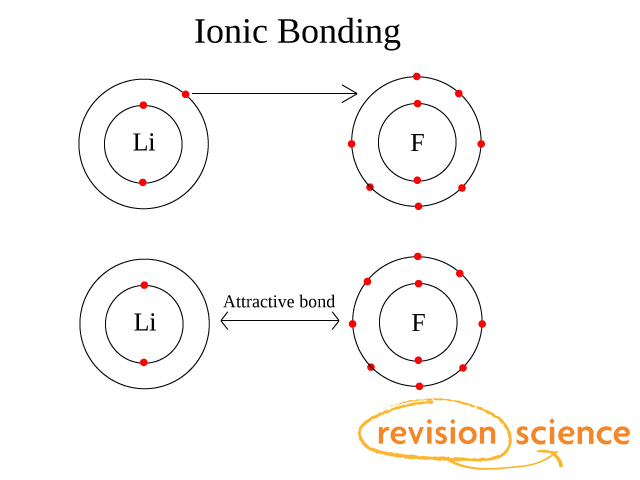
Ionic Bonding
Atoms lose or gain electrons to attain a complete outer shell of electrons. The charged atoms are called ions. When atoms lose electrons they become positive ions, when they gain electrons they become negative ions. It is the electrostatic forces of attraction which hold the ions together.
Metallic Bonding
In metals, atoms are closely packed together. They are so close that the outer shells of electrons on neighbouring atoms overlap. This means that the electrons are able to travel around the whole of the metal. This is called delocalisation. The electrons are sometimes described as being in a delocalised sea of electrons.
Since the electrons are free to move through the structure, metals conduct electricity.

Ionic and Covalent Bonding is NOT black and white
Polarisation is a scientific sounding word but it is important to understand what it means. It can be applied to two kinds of bonding and has different meanings in each case.
In the context of covalent bonding:
- In reality, not all bonds are perfectly covalent. To explain why we have to define a concept called electronegativity.
- Electronegativity is the ability of an atom to pull electrons to itself in a covalent bond.
- In hydrogen fluoride the fluorine atoms are much more electronegative than the hydrogen. It pulls the electrons toward it creating what is called a polar bond.
- Molecules with asymmetric charge distribution are said to be polar molecules and possess a dipole.
In the context of ionic bonding:
- Polarisation in ionic bonds refers to the electron cloud distortion of the ions.
- In the perfect ionic model, the ions would be perfectly spherical and the positive and negative ions would attract each other but not distort each other. Reality is a bit different because the positive ion will pull some of the electron cloud of the negative ion towards itself.
- If a cation is highly charged it will exert a strong electrostatic attraction on the anion and distort the electron cloud.
- If the anion has a large electron cloud it will be easily distorted.
- If the electron cloud is distorted there will be electron density between the two ions giving the bond some covalent character.
- These points are summarised in Fajans rules.
At GCSE there was a simple black and white choice between ionic and covalent. If covalent bonds can have unfair sharing of electrons and ionic bonds can have some covalent character, then there is a grey scale between the two extremes.
An interesting example is to consider aluminium fluoride and aluminium chloride.
Assume they are covalent.
- The electronegativity difference is bigger in aluminium chloride and so that would be more covalent.
Assume they are ionic.
- The chloride ion will be more distorted than the fluoride ion and so the chloride is more covalent.
