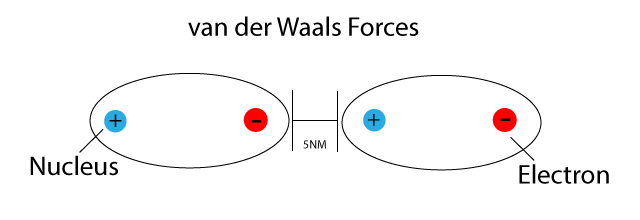
van der Waals Forces
Van der Waals forces are induced dipole-dipole forces.
These forces exist between all molecules poler, or non-polar.
They are weak intermolecular forces caused by attractions between very small dipoles in molecules.
As the number of electrons increases so does the size of the oscillating and induced dipoles, the size of the attractive forces between the molecules, and the size of the van der Waals forces.
Image