Equilibria
To apply calculations to an equilibrium, consider the general reversible reaction:
aA + bB in equilibrium with cC + dD
An equilibrium constant is defined as Kc = [C]c[D]d / [A]a[B]b
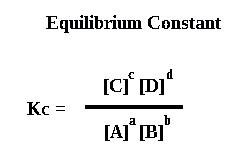
Products are on the top line, with reactants on the bottom line.. The square brackets, [ ], mean concentration in moldm-3.
The lowercase letters are the number of moles of each substance.
To work out the units of your result, replace A, B, C, and D with moldm-3 and simplify the expression.

In this example each substance has 1 mole and the units then cancel out.
If the reaction involves gases, partial pressures are used instead of concentrations and the equilibrium constant is called Kp . The layout is the same (except for a change in bracket type) and the powers come from the big numbers again.
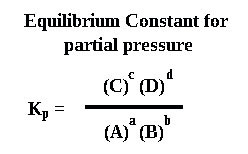
It is a common silly mistake to mix up "p" and "c" and what type of bracket to use.
Using "p" and "( )" for a concentration question or the other way round will cost you a lot of marks.
The units in a Kp calculation are kPa and for the result must be worked out using the same method to work out the units for Kc.
Kc and Kp can only be changed by changing temperature.
An equilibrium will often be shown with a ΔH value. This is for the forward (left to right) direction.
If ΔH is positive (endothermic in the forward direction), Kc increases with increasing temperature.
This means that more products are formed.
Some people get confused by trying to learn all the possible combinations of increase and decrease, endothermic and exothermic. Learn one of the them confidently and thenwork out the other possibilities if you need to do so.
Increasing the concentration of one of the reactants does not change the value of Kc but does mean that the concentration of products must also increase.
Increasing the partial pressure of one of the reactants does not change the value of Kp but does mean that the partial pressure of products must also increase.
Tips on dealing with equilibrium calculations
Equilibrium calculations can be difficult for two main reasons. Either you find it difficult to do the mathematical juggling or else you find it difficult to understand what the examiner is telling you in the question. Of course if you are really unlucky you might find both bits difficult.
The best first advice is to write your answer neatly. In this way both you and the exam marker will be able to understand what you have achieved. You will find the calculation a bit easier and the marker might be able to give you some credit for a partial answer.
Consider a fairly basic question such as: 6 moles of PCl5 are allowed to come to equilibrium with PCl3 and Cl2. The total volume of the container is 5 dm3. At equilibrium, it is found that there are only 4 moles of PCl5 left. Calculate the value of Kc and give its units.
Let’s put that into the table along with the fact that we will say x moles of PCl5 will decompose to give the equilibrium mixture.
Equation PCl5 PCl3 Cl2
Initial moles 6 0 0
Equilibrium moles 6 – x x x
Equilibrium concentration (6 – x) / 5 x / 5 x / 5
The question tells us that at equilibrium, only 4 moles of PCl5 are left.
This means that 6 – x = 4 and so x = 2.
Let’s modify the table to include this information.
Equation PCl5 PCl3 Cl2
Initial moles 6 0 0
Equilibrium moles 4 2 2
Equilibrium concentration 4 / 5 2 / 5 2 / 5
Kc = [PCl3][Cl2] / [PCl5] and so we can add the values that we know:
Kc = (2/5) (2/5) / (4/5) solving this gives Kc = 0.2
The units are (mol dm-3) x (mol dm-3) / (mol dm-3) and so units are mol dm-3
More complications can be that:
• The “amounts” information is given as masses etc.
Simply use GCSE moles calculations to convert it to moles.
• The chemical equation is not a simple “one-to-one” relationship between the reagents and products. Put the appropriate “big numbers” in the Kc equation as powers.
Think about how you modify “x” in the “equilibrium moles” line of the grid.
As an example of this consider:
2 moles of N2 and 5 moles of H2 are mixed with 4 moles of NH3 and allowed to come to equilibrium in a container of total volume = 3 dm3. At equilibrium, there are 6 moles of NH3. Calculate the value of Kc at this temperature and give its units.
Equation N2 3H2 2NH3
Initial moles 2 5 4
Equilibrium moles 2 - x 5 – 3x 4 + 2x
Equilibrium concentration (2 – x)/V (5 – 3x)/V (4 + 2x)/V
But we can add to this since we know that 4 + 2x = 6. x = 1 Modify the table:
Equation N2 3H2 2NH3
Initial moles 2 5 4
Equilibrium moles 1 2 6
Equilibrium concentration 1/V 2/V 6//V
Kc = (1/3) x (2/3)3 / (6/3)2 = 0.025
Units are mol2dm-6
