Boltzmann Distribution
A Boltzmann Distribution shows the distribution of molecular energies in a gas at constant temperature.
Image
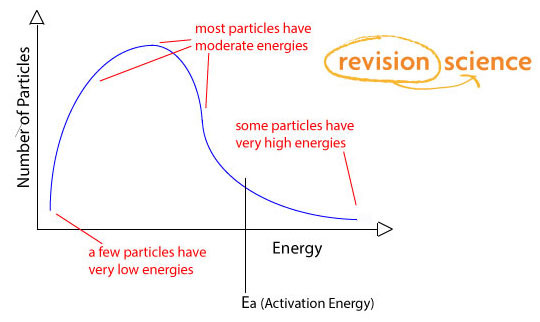
Most gas molecules have energies within a comparatively narrow range.
• The curve will only meet the energy axis at infinity energy. No molecules have zero energy.
• The area under the curve gives the total number of gas molecules.
• Only those molecules with more energy than the activation energy of the reaction are able to react.
