Born-Haber Cycles
A lattice enthalpy is the enthalpy change when a mole of solid ionic compound forms from gaseous ions.
Lattice enthalpies can't be directly calculated but using a Born-Haber cycle you can go through the different bond enthalpy changes to get a result.
Below is a Born-Haber Cycle for the formation of Magnesium Chloride (actual figures have been omitted for brevity).
Image
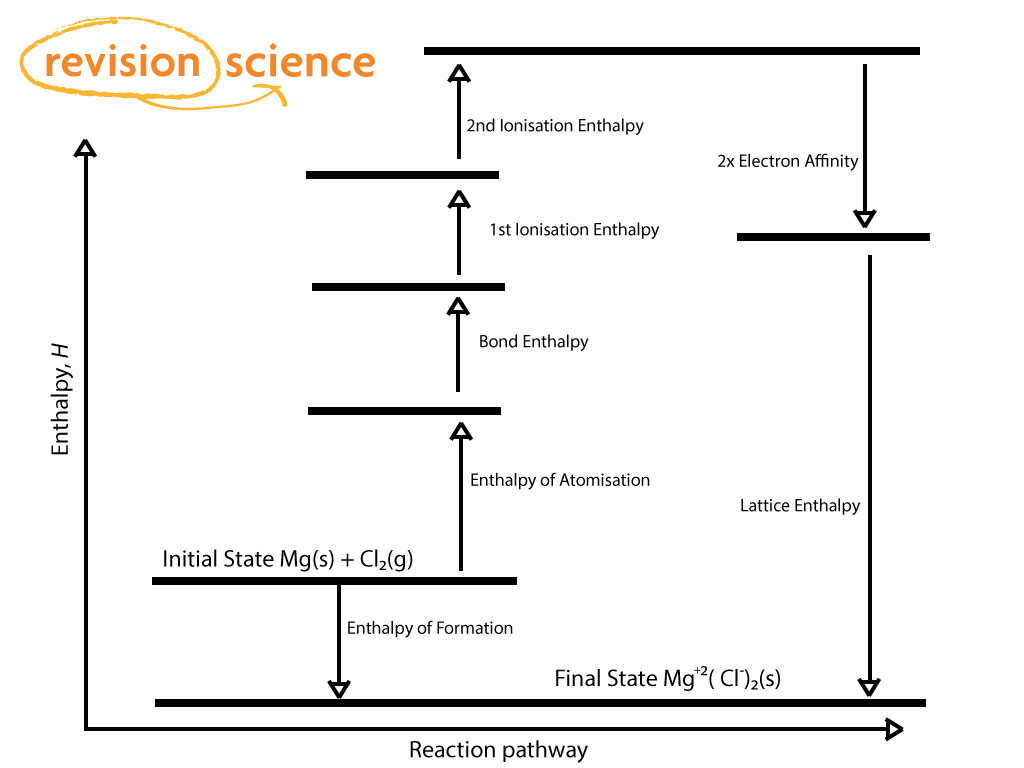
From Hess' Law
Enthalpy of Formation = The Sum of all the other Enthalpies going from Atomisation to Lattice Enthalpy.
The calculated value will be different from the experimental value as positive and negative ions are not exactly spherical.
