Catalysts
Catalysts change the rate of chemical reactions but are unchanged at the end of a reaction. Most speed up the rate but some, called inhibitors, slow it down.
Energy Profile diagram
Image
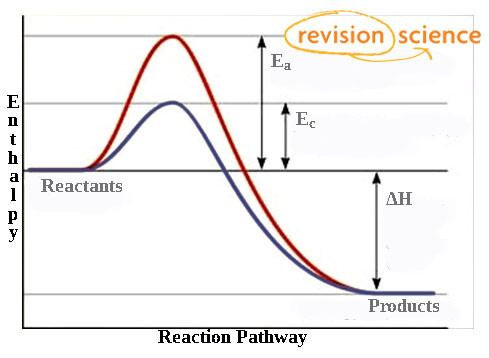
Activation energy without catalyst (Ea) is higher than with catalyst (Ec).
Boltzmann distribution
Image
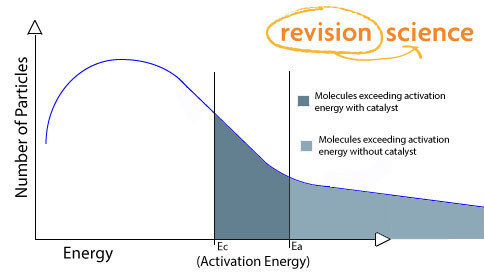
The catalyst does not change the distribution curve but a greater number of particles now surpass the activation energy (Ec).
