Free-Energy Change & Entropy Change
Free-Energy Change
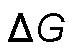
and Entropy Change
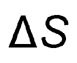

, whilst important, is not sufficient to explain spontaneous endothermic
The concept of increasing disorder (entropy change) accounts for this phenomenon.
E.g. the production of CO2 from hydrogencarbonates with acid is endothermic but occur without heating, because the products are significantly more disordered than the products.
The balance between entropy and enthalpy determines the feasibility of a reaction and is called the change in free energy.
(derivation not required).
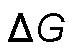
must be negative for a reaction to proceed spontaneously
Clarifying that the thermodynamic definition of Entropy requires a reversible system.
This video discusses what entropy is and what it isn't.
This video looks at Maxwell's Demon: A thought experiment that seems to defy the 2nd Law of Thermodynamics
