Hess’s Law and Hess Cycles
Hess's Law can be considered an extension of the Law of Thermodynamics. It states that if a reaction can take place by more than one route from the same initial and final conditions then the total enthalpy change is the same for each route.
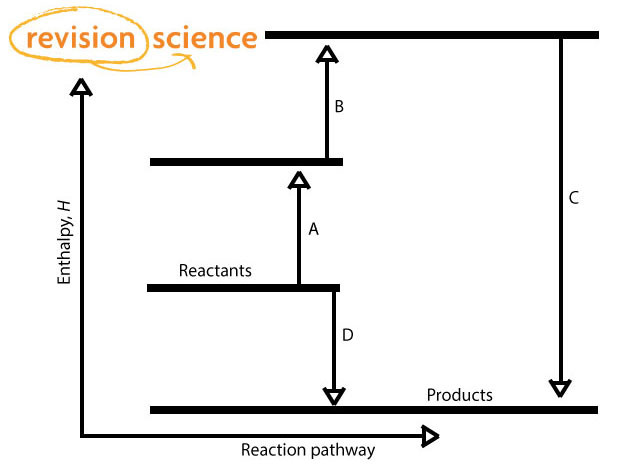
There are 2 routes:
A + B + C
D
By Hess' Law: A + B + C = D
If 3 of the above values are known then the 4th can be worked out.
A Hess Cycle can be used to work out Enthalpy Changes.
There are textbooks filled with values for Enthalpies of Formation for various compounds.
You can use Hess's Law along with these values to work out enthalpy changes for many kinds of reactions.
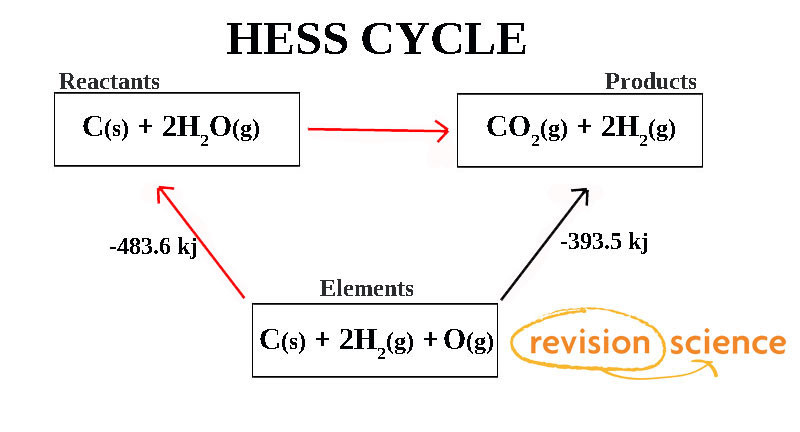
In the above diagram the Enthalpy change along the Red path is the same as along the Black Path.
To work out the Enthalpy change of the reaction is then:
ΔHf = Sum of Products – Sum of Reactants.
ΔHf = -393.5 - (-483.6) = 90.1 kj.
As the answer is positive the reaction is Endothermic.
This video shows how to use Hess's Law and standard heats of formation to determine the enthalpy change for reactions.
