Stereoisomerism
There are two types of stereoisomerism to deal with.
Geometric isomerism
Requires a carbon to carbon double bond. It must have two different groups on the "left hand" carbon atom and two different groups on the "right hand" carbon atom.
It doesn't matter about any similarity or not between the right hand side and the left hand side.
The two forms are known as "cis" and "trans" isomers. They are also known as "Z" and "E" isomers.
Geometric isomerism is a specific form of stereoisomerism.
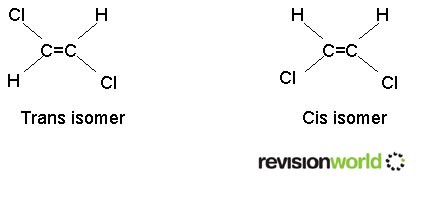
This form of isomerism arises because there is no rotation about the double bond
Optical isomerism
Requires a carbon atom to have four differnt groups on it. This is known as a chiral carbon atom. A carbon atom with four different groups on it will have two non-superimposable mirror images.
A diagram will be added later but for now, think about your hands...they are mirror images but you cannot make your left hand match your right hand exactly.
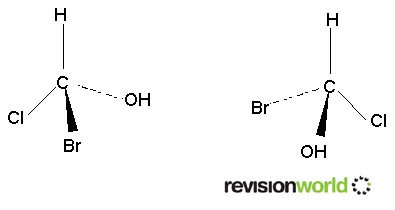
These two molecules cannot be superimposed. If you don’t believe it build a model.
This form of isomerism is called optical isomerism because the different isomers can rotate polarised light in different directions
The central carbon is called a chiral carbon.
The two isomers are called enantiomers.
A mixture of isomers is called a racemic mixture
This video explains about Optical Isomerism
