Alkenes
After studying this section you should be able to:
- recognise the nature of cis-trans isomerism in alkenes
- understand that the double bond in alkenes takes part in addition reactions with electrophiles
- understand the use of alkenes in the production of organic compounds
- understand what is meant by addition polymerisation
Alkenes
Alkenes are unsaturated compounds with a C=C double bond. The high electron density of the double bond makes alkenes more reactive than alkanes.
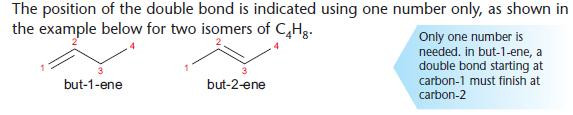
Positional isomerism
A type of structural isomerism in which the functional group is in a different position on the carbon skeleton.
What is a double bond?
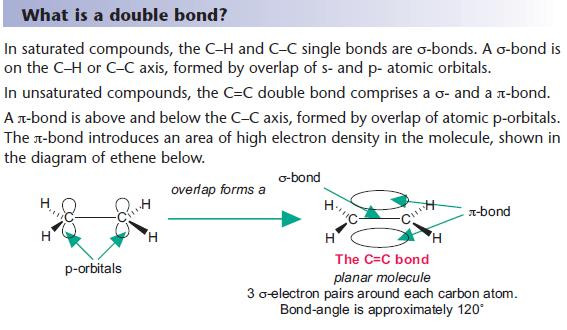
Molecular Orbitals
σ- and π-bonds are molecular orbitals – these bond together the atoms in a molecule.
cis-trans (or geometric) isomerism
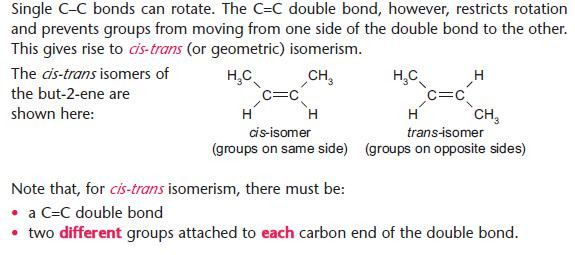
See also structural isomerism,
No rotation possible about a C=C bond.
These are relatively easy exam marks. Make sure that you learn this thoroughly.
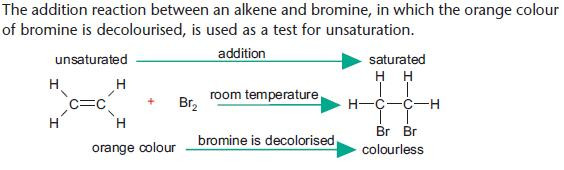
Addition reactions of alkenes
An addition reaction of an alkene involves the opening of the double bond with the formation of a saturated addition product.

Addition of bromine
Mechanism of electrophilic addition (not EDEXCEL)
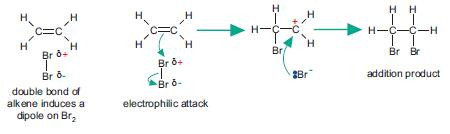
Be careful with the direction of the curly arrows.
Don’t get confused by the charges and partial charges within this mechanism.

Remember that a curly arrow shows the movement of an electron pair.
Addition using acidified manganate(VII)
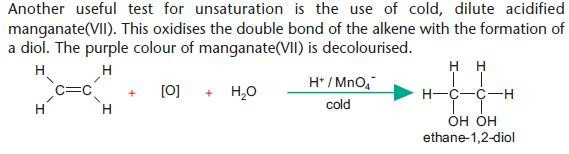
Note that [O] is used in equations to represent an oxidising agent. Many candidates in exams get this reaction wrong by
incorrectly adding Mn. KMnO4 is used as a source of manganate (VII) ions.
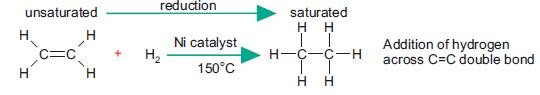
Reduction with hydrogen

Alkenes are reduced when treated with hydrogen gas in the presence of a nickel catalyst at 150°C. The process of adding hydrogen across a double bond is sometimes referred to as hydrogenation.
Further addition reactions of ethene
Addition reactions of alkenes are useful in organic synthesis. Some further addition reactions of ethene are shown in the flowchart below.
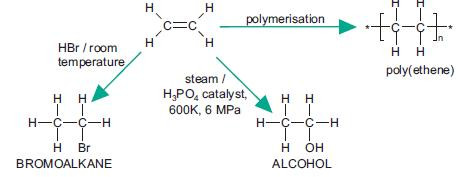
Hydrogenation of C=C is used in the production of solid margarine from unsaturated liquid vegetable oils. Saturated
fats are solid and the amount of hydrogenation can be controlled to give the correct texture of margarine.

Addition reactions of unsymmetrical alkenes
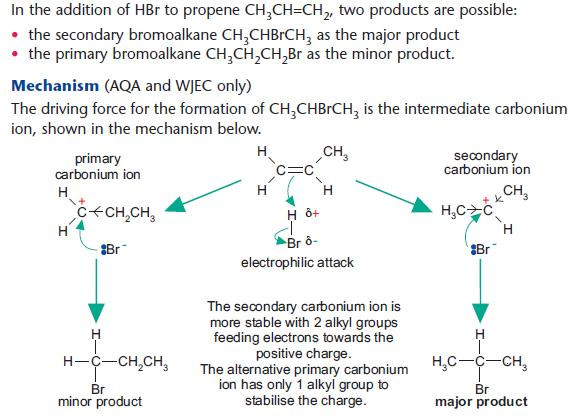

Addition polymerisation of alkenes
An addition polymer is a long-chain molecule with a high molecular mass, made by joining together many small molecules called monomers:
MANY MONOMERS → SINGLE POLYMER
n molecules → 1 single molecule
- The monomer used is an unsaturated alkene containing a double C=C bond.
- The addition polymer formed is a saturated compound without a double bond.
Many different addition polymers can be formed by using different alkene monomer units, based on the ethene molecule.
The equations below shows the formation of poly(ethene), poly(chloroethene) (pvc) and poly(phenylethene) (polystyrene).
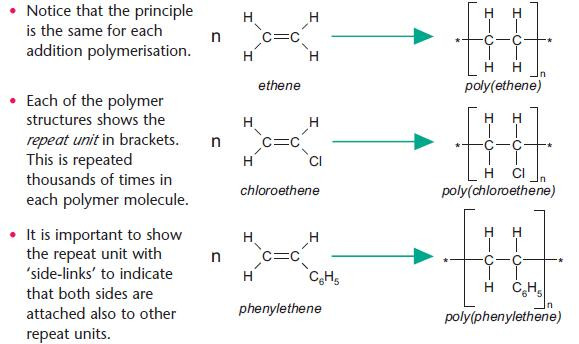
You should be able to draw a short section of a polymer given the monomer units (and vice versa).
Make sure that you can show the repeat unit of a polymer.
Properties of monomers and polymers
- The monomers are volatile liquids or gases.
- Polymers are solids.
- This difference in physical properties is explained by increased van der Waals’ forces between the much larger polymer molecules
Problems with disposal
- Addition polymers are non-biodegradable and take many years to break down.
- Disposal by burning can produce toxic fumes (e.g. depolymerisation produces monomers; dioxins from combustion of chlorinated polymers).
Rather than simply disposing of waste polymers, there is now movement towards recycling, combustion for energy production, and use as a feedstock for cracking.
This eliminates problems with disposal and helps to preserve finite energy resources.
Progress Check

Epoxyyethane, (CH2) 2O

Epoxyethane is prepared by partial oxidation of ethene.
Reactions of epoxyethane
The three-membered ring in epoxyethane is strained making the molecule highly reactive towards nucleophiles such as water and alcohols. Reaction brings about ring opening.
Hydrolysis of epoxyethane
Much of the epoxyethane produced is converted into ethane-1,2-diol which is used directly as antifreeze and indirectly to make polyesters such as Terylene. In industry, this is done by treating epoxyethane with an excess of water at 60°C in the presence of sulphuric acid.
Hazards from preparing and handling epoxyethane
- Epoxyethane is a colourless gas, b.p. 10 °C, which is both flammable and explosive.
- Epoxyethane is also toxic and may cause irritation to the respiratory system and neurological effects.
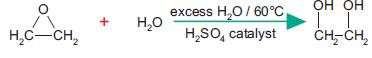
The resulting aqueous solution is concentrated to 70% by evaporation and then purified by fractional distillation. The properties of ethane-1,2-diol make it ideal for antifreeze:
- low melting point (–12°C)
- high boiling point (198°C)
- complete miscibility with water (with which it readily forms hydrogen bonds).
Ethane-1,2-diol is also used in the manufacture of polyesters, such as Terylene.
