Functional groups
Saturated hydrocarbon chains are comparatively unreactive. The reactivity is increased by the presence of a functional group – the reactive part of a carbon compound.
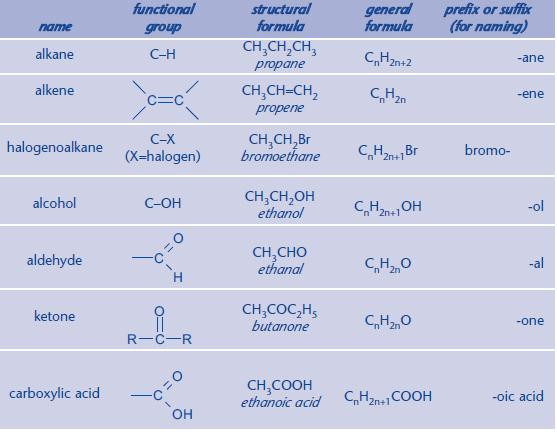
Common functional groups
It is essential that you can instantly identify a functional group within a molecule. You can then apply the relevant chemistry. If you continue to study Chemistry to A Level, you will meet more functional groups.
The main functional groups for AS Chemistry are shown below.
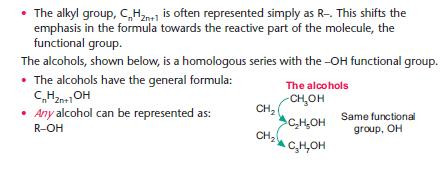
• When studying reactions of a homologous series, the alkyl group is relatively unimportant – it is the functional group that reacts.
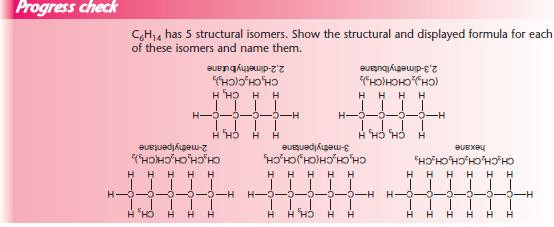
With so many isomers possible, it is important that each has an individual name. The examples below show the steps needed to name an organic compound.
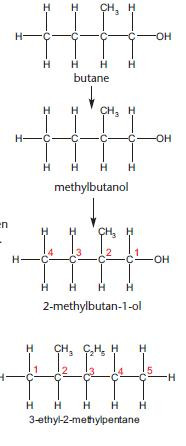
Rule 1
• The name is based upon the longest carbon chain on an alkane.
Rule 2
• Any functional groups and alkyl groups are identified.
• These are then added to the name as a prefix (e.g. chloro-) or suffix (e.g. -ol)
Rule 3
If there is more than one possible isomer then the carbon atoms are labelled with numbers. Numbering starts from the end giving the lowest possible numbers for any functional groups and side chains. In this example, numbering from the left would give the incorrect name of 3-methylbutan-4-ol.
Rule 4
If there is more than one alkyl or functional group, they are placed in alphabetical order. This rule is not needed for the example above but it does need to be applied to name the compound below.
Types of bond fission
The breaking of a covalent bond is called bond fission. Reactions of organic compounds involve bond fission followed by the formation of new bonds. Two types of bond fission are possible, homolytic fission and heterolytic fission.
These are very important principles, used throughout organic chemistry.

- In the example above, a covalent bond breaks so that one of the bonding electrons goes to each of A and B.
- Homolytic fission forms two free-radicals.
- A free radical is a species with an unpaired electron
Heterolytic fission

- In the example above, a covalent bond breaks so that both the bonding electrons go to either A or B.
- Heterolytic fission forms oppositely-charged ions.
NOTE:
Homolytic fission → free radicals
Heterolytic fission → ions
Percentage yields
Organic reactions typically produce a lower yield than expected from the balanced equation.
KEY POINT: The yield is usually expressed as a percentage yield: percentage yield = actual yield / theoretical yield × 100%
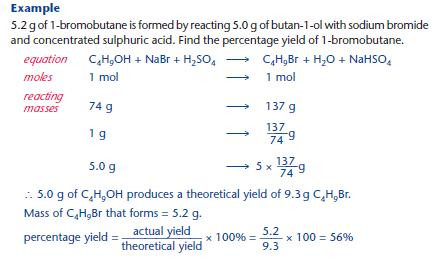
Factors that can contribute to a low yield:
Organic reactions often do not go to completion.
An organic compoundoften reacts to produce mixture of products.
The purification stages result in loss of some of the desired product.