Hydrocarbons from oil
Useful products from crude oil
Crude oil is a fossil fuel, formed from the decay of sea creatures over millions of years. It is a complex mixture of hydrocarbons, containing mainly alkanes. Crude oil cannot be used directly but it is the source of many useful products.
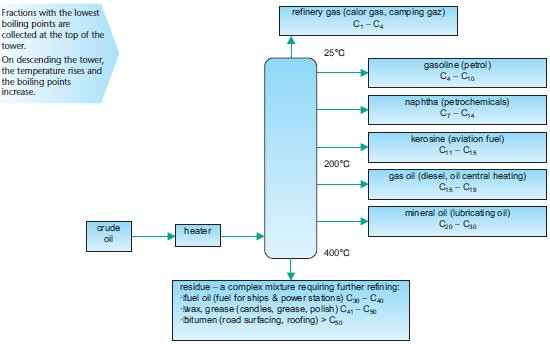
The first stages in the processing of crude oil are described below. Fractional distillationCrude oil is heated and passed into a fractionating tower, separating the complex mixture into fractions. Note that the fractions are not pure and contain mixtures of hydrocarbons within a range of boiling points. The fractionating tower, shown below, shows the temperature gradient which separates the fractions according to their boiling points.
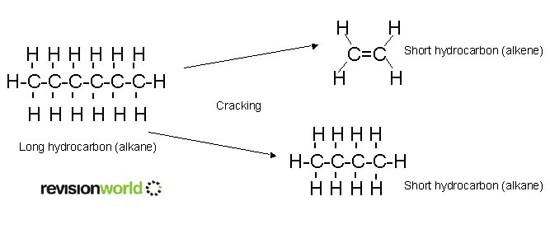
Cracking
Cracking is the breaking down of an unsaturated hydrocarbon into smaller hydrocarbons. The purpose of cracking is to produce high demand hydrocarbons:
- short-chain alkanes for use in petrol
- alkenes, as a feedstock for a wide range of organic chemicals, including polymers
Cracking is the starting point for the manufacture of many organics.
Thermal cracking heats the naphtha fraction with steam at a high temperature (about 800°C) and high pressure. This forms a mixture of straight-chain alkanes and alkenes (mainly ethene) with a small proportion of branched and cyclic hydrocarbons. Some hydrogen is also produced.
The following equations summarise the overall process of cracking.
Thermal cracking breaks C-C bonds by homolytic fission forming free radicals.
Catalytic cracking breaks C-C bonds by heterolytic fission forming ions.
Catalytic cracking processes heavy long-chain fractions obtained in larger quantities than required. A mixture of the vapourised fraction and a zeolite catalyst are reacted at about 450°C using a slight pressure only. The process forms a higher proportion of branched and cyclic hydrocarbons than thermal cracking (see also reforming and isomerisation below). These are used for petrol.
Reforming
Reforming uses heat and pressure to convert unbranched fractions into cycloalkanes (e.g. cyclohexane) and arenes (e.g. benzene). These products are used in petrol and as a feedstock for a wide range of organic chemicals including many pharmaceuticals and dyes.After studying this section you should be able to:
- understand how hydrocarbon fractions are separated from crude oil
- describe how low demand fractions are processed into hydrocarbons of higher demand
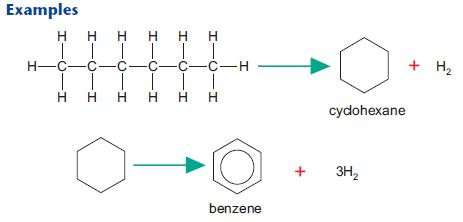
The use of leaded petrol has been phased out in the UK. Unleaded petrol and lead replacement petrol require a higher
proportion of branched and cyclic hydrocarbons for effective combustion. This has increased demand for reformed alkanes.
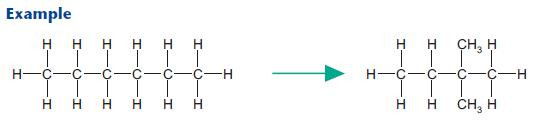
Isomerisation
Isomerisation converts unbranched hydrocarbons into branched hydrocarbons, needed for petrol.
Progress Check

