Introduction to Organic Chemistry
The following topics are covered in this chapter:
- Basic concepts
- Alkenes
- Hydrocarbons from oil
- Alcohols
- Alkane
- Halogenoalkanes
Basic concepts
- After studying this section you should be able to:
- understand the different types of formula used for organic compound
- understand the terms saturated and unsaturated hydrocarbon
- understand what is meant by structural isomerism
- recognise the alkanes and alkyl groups
- understand what is meant by a homologous series
- recognise common functional groups
- apply rules for naming simple organic compounds
- understand the difference between homolytic and heterolytic fission
- calculate percentage yields
Types of formula
In organic chemistry, there are many ways of representing a formula.
For the compound butane, with 4 carbon atoms and 10 hydrogen atoms:
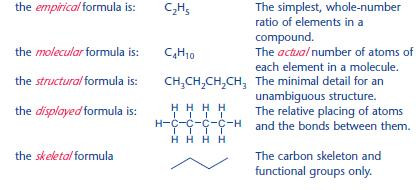
Carbon chains
Hydrocarbons
Hydrocarbons are compounds of carbon and hydrogen only.
-
A saturated hydrocarbon has single bonds only.
-
An unsaturated hydrocarbon contains a multiple carbon carbon bond.

Alkanes
Carbon atoms can bond with other carbon atoms to form an enormous range of compounds with different carbon-chain lengths. The simplest organic compounds are a family of saturated hydrocarbons called the alkanes, shown below.
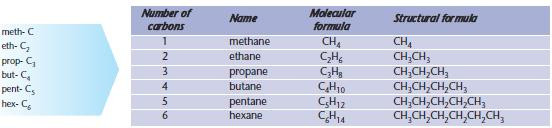
Note the following points.
- The name of the alkane ends with –ane.
- The prefixes (meth-, eth-, …) are used to represent the number of carbon atoms. You will need to use these many times in organic chemistry and they must be learnt.
General formula

Homologous series

Note that physical properties, such as boiling point and density, do gradually change as the length of the carbon chain increases.
