Excitation and Ionisation
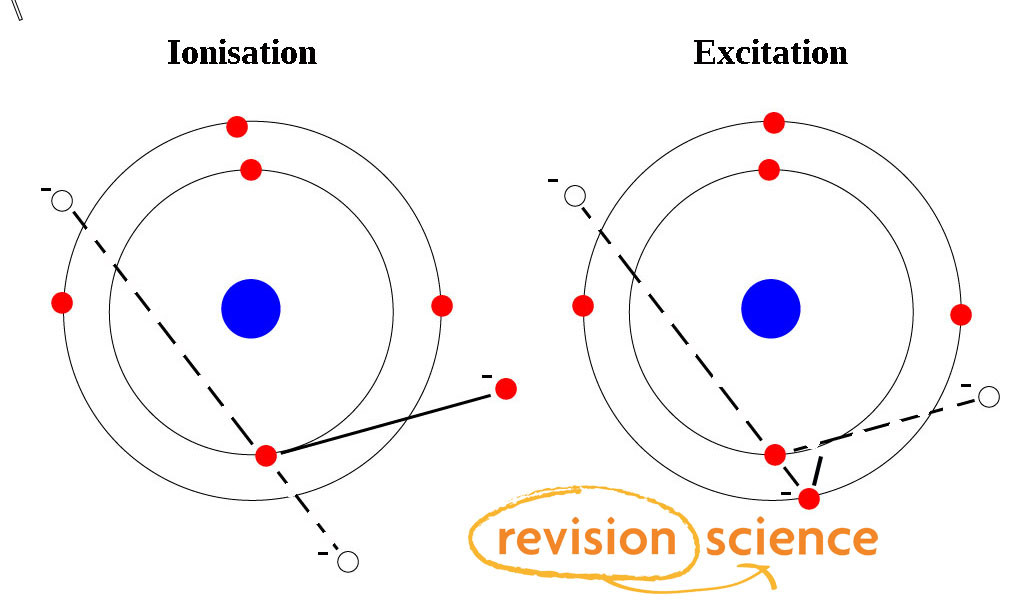
Excitation
Excitation is the result of energy being given to an electron moving it to a higher energy level.
It will still orbit the nucleus, unless the electron energy level is greater than the ionisation energy level of the atom.
Ionisation
If enough energy is given to the electron to remove it from the atom ionisation has occurred.
In fluorescent tubes a gas has an electric current pass though it. This causes electrons to become excited and ionise. When electrons return towards the ground state they lose energy which is emitted as UV radiation. This radiation is absorbed by the coating in the tube which emits visible light.
Image