Photoelectric Effect
The Photoelectric effect provides evidence that electromagnetic waves have particle-like behaviour.
In the photoelectric effect, electrons are emitted from a metal’s surface when it absorbs electromagnetic radiation.
The diagram below shows this.
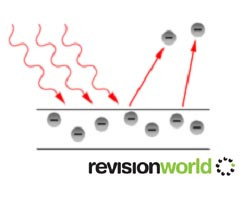
There are no electrons emitted below a certain frequency, called the threshold frequence, fo , which is different for different metals.
Above this frequency, electrons are emitted with a range of kinetic energies up to a maximum, (½mv2)max
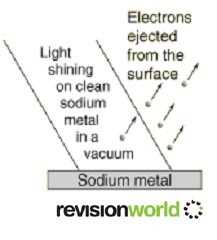
The wave model cannot explain the Photoelectric effect.
The explanation for this relies on the concept of the photon, a quantum packet of energy. So EM radiation is given by short bursts of energy.
The relationship between the energy of the photon, E, and its frequency is given by:
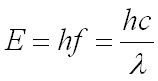
E = energy of the photon in joules, J
h = the Plank constant 6.63 x 10-34Js
f = frequency in hertz, Hz
c = speed of light 3.00 x 108 metres per second, ms-1
λ= wavelength in metres, m
The energy of a photon can be measured in Joules or Electronvolts.
One electronvolt is the energy transfer when an electron moves through a potential difference of 1 volt, given by: 1 eV = 1.60x10-19 J
The minimum energy required for the electron to escape the metal is called the work function, Φ
If energy is less than Φ, then no emission occurs
Emission is possible when hf = Φ
The photoelectric equation relates the maximum kinetic energy of the emitted electrons to the work function and the energy of each photon: hf = Φ + (½mv2)
At the threshold frequency, the minimum frequency that can cause an emission is zero, so the equation becomes: hfo = Φ
