Discovery and Development of Drugs
This section explains the discovery and development of drugs. The discovery and development of drugs is a crucial area of medical science. Drugs can be sourced from natural substances, such as plants and microorganisms, or developed synthetically in laboratories. Once discovered, drugs must go through a series of stages, including rigorous clinical trials, to ensure their safety and effectiveness before they can be used by the general population.
In the past, many drugs were discovered by chance or through traditional knowledge of plants and natural remedies. Today, the process involves a combination of scientific research, testing, and technological advancement.
Drugs from Natural Sources
Many drugs have been discovered from plants, animals, or microorganisms. These natural substances are often found to have therapeutic properties and are then developed into medicines. Below is a table of some notable drugs, their origins, and uses:
| Drug Name | Where it was Found/Origin | Use |
|---|---|---|
| Digitalis | Derived from the foxglove plant (Digitalis purpurea) | Used to treat heart failure and arrhythmias (irregular heartbeats) by improving the efficiency of the heart. |
| Aspirin | Derived from the bark of the willow tree (Salix alba) | Used as an anti-inflammatory, pain reliever, and to reduce fever. It is also used to reduce the risk of heart attacks and strokes. |
| Penicillin | Discovered from the mould Penicillium by Alexander Flemming. | Used to treat bacterial infections. It was the first antibiotic discovered and revolutionised medicine. |
Modern Drugs
Modern drugs are often developed through a combination of laboratory research, testing, and the application of biotechnology. Advances in genetics and biochemistry have made it possible to design drugs that target specific pathways in the body, providing more effective treatments with fewer side effects.
The development of new drugs often involves the following stages:
- Discovery: Scientists identify potential compounds through research, which could involve screening thousands of substances.
- Preclinical Testing: The drug is tested on animals to assess its safety and effectiveness.
- Clinical Trials: The drug is tested on human volunteers in several phases to ensure its safety, effectiveness, and appropriate dosage.
- Regulatory Approval: If the drug passes clinical trials, it is reviewed by regulatory bodies (such as the MHRA in the UK) before being approved for public use.
Clinical Trials
Clinical trials are essential in the development of new drugs and involve testing a new drug or treatment on human participants. These trials are conducted in several phases to evaluate the safety, efficacy, and potential side effects of a drug.
Phase 1: The drug is tested on a small group of healthy volunteers to assess its safety and identify any potential side effects.
Phase 2: The drug is tested on a larger group of people who have the condition it is intended to treat, to evaluate its effectiveness and further assess its safety.
Phase 3: The drug is tested on an even larger group of people, including diverse populations, to confirm its effectiveness, monitor side effects, and compare it to existing treatments.
Phase 4: Post-marketing surveillance occurs, where the drug is monitored for long-term side effects once it is available on the market.
Monoclonal Antibodies
Monoclonal antibodies (mAbs) are laboratory-made molecules designed to mimic the immune system's ability to fight off harmful pathogens such as viruses and bacteria. They are produced by creating identical copies (clones) of a single type of antibody, hence the term "monoclonal."
Monoclonal antibodies are used in the treatment of various conditions, including cancers, autoimmune diseases, and infections. They can be designed to target specific cells, such as cancer cells, and bind to them to destroy or inhibit their growth.
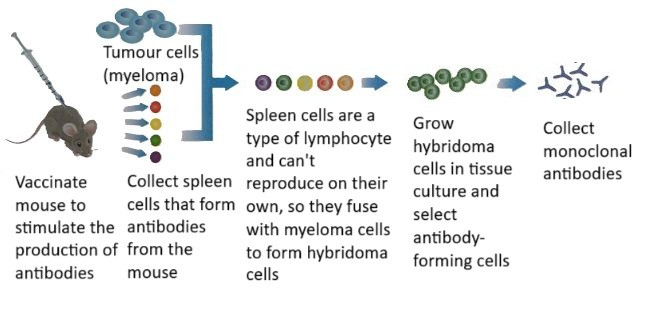
Uses of Monoclonal Antibodies
Monoclonal antibodies are used in various medical applications, including:
- Cancer treatment: Targeting cancer cells to kill or inhibit their growth.
- Autoimmune diseases: Treating conditions like rheumatoid arthritis by targeting and neutralising the molecules involved in the autoimmune response.
- Infectious diseases: Monoclonal antibodies can be used to treat infections by targeting the specific pathogen.
- Diagnostic tests: Used in pregnancy tests and other diagnostic tools to detect the presence of specific substances.
Table: Monoclonal Antibodies
| Monoclonal Antibody | Use | How It Works | Advantages |
|---|---|---|---|
| Rituximab | Treatment of certain cancers (e.g., lymphoma) and autoimmune diseases (e.g., rheumatoid arthritis) | Targets and binds to CD20 on B cells, which are involved in the growth of cancers like lymphoma, and helps the immune system destroy them. | Targeted treatment with fewer side effects compared to traditional chemotherapy. |
| Trastuzumab (Herceptin) | Treatment of HER2-positive breast cancer | Binds to the HER2 protein on cancer cells, preventing the growth of cancer and promoting immune-mediated destruction. | Improves survival rates for HER2-positive breast cancer patients. |
| Adalimumab (Humira) | Treatment of autoimmune diseases like rheumatoid arthritis and Crohn's disease | Targets and neutralises TNF-α, a molecule involved in inflammation, reducing inflammation and symptoms. | Reduces inflammation and improves quality of life in autoimmune conditions. |
| Palivizumab | Prevention of respiratory syncytial virus (RSV) infection in infants | Binds to the RSV virus, preventing it from infecting healthy cells. | Prevents severe RSV infections in high-risk infants. |
Advantages of Monoclonal Antibodies
- Specificity: Monoclonal antibodies can be designed to target a specific protein or cell, reducing the impact on healthy cells and tissues, leading to fewer side effects.
- Effectiveness: They can be used to treat diseases that were previously difficult to treat, such as some types of cancer and autoimmune diseases.
- Personalised Medicine: They can be tailored to target specific individuals' disease characteristics, making treatments more personalised and effective.
Summary
The development of drugs has evolved from the use of naturally sourced substances like Digitalis, Aspirin, and Penicillin, to the creation of modern drugs through scientific research and biotechnology. Clinical trials are crucial to ensuring the safety and effectiveness of new drugs before they are approved for use. Monoclonal antibodies represent a significant advancement in treating specific diseases, offering targeted therapies with fewer side effects. Together, these innovations continue to improve healthcare and the treatment of diseases worldwide.
