Enzymes
Enzymes are biological catalysts – protein molecules that speed up chemical reactions. They catalyse chemical reactions in living cells such. Examples of these chemical reactions are:
- Respiration
- Photosynthesis
- Protein synthesis
Active Sites
The shape of an enzyme determines how it works. Enzymes have active sites that substrate molecules (the substances involved in the chemical reaction) fit into when a reaction happens.
The active site has to be the right shape for the substrate molecules to fit into. This means that enzymes have a high specificity for their substrate – a particular type of enzyme will only work with one or a smaller number of substrates. The animation shows how this works:
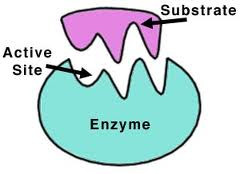
The mechanism involved is called the 'lock and key' mechanism. Just as a lock will only accept one key, an enzyme will only accept one substrate.
Enzyme-catalysed reactions
Enzymes work best at particular temperatures and pH values.
Enzymes and Temperature
At low temperatures, enzyme reactions are slow. They speed up as the temperature rises until an optimum temperature is reached. After this point the reaction will slow down and eventually stop. The graph shows what happens to enzyme activity when the temperature changes.
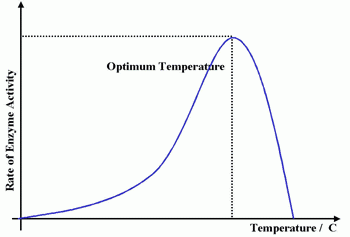
In the example above, enzyme activity increases steadily. It peaks (the enzyme's optimum temperature) then decreases rapidly.
Enzymes and pH
Different enzymes work best at different pH values, their optimum pH. Many enzymes work fastest in neutral conditions. Making the solution more acidic or alkaline will slow the reaction down. At extremes of pH the reaction will stop altogether.
Some enzymes, such as those used in digestion, are adapted to work faster in unusual pH conditions. For example, stomach enzymes have an optimum pH of 2, which is very acidic.
The graph shows what happens to enzyme activity when the pH changes.
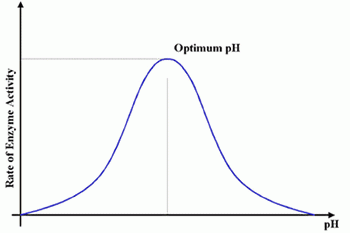
In the example above, enzyme activity increases, it peaks then decreases.
