Identification of Cations
In this section, we will look at how to identify different metal ions, or cations, using two key methods: flame tests and precipitation reactions with sodium hydroxide (NaOH). These techniques are essential for chemical analysis, as they allow chemists to determine the presence of specific metal ions in a sample.
Flame Tests to Identify Metal Ions
Flame tests are a simple and effective way to identify certain metal ions by observing the colour of the flame produced when a sample is heated.
The Test:
- A sample of the metal salt (often in the form of a metal chloride) is placed on a wire (usually a platinum or nichrome wire) that has been cleaned by dipping it into hydrochloric acid and heating it in a Bunsen burner flame.
- The metal salt is then heated in the Bunsen flame, and the characteristic colour of the flame can help identify the metal ion present.
Colours of the Bunsen Flame for Different Metals
Each metal ion produces a distinctive colour when heated in a flame. The colour arises due to the excitation of electrons in the metal ions, which then release energy in the form of visible light when they return to their ground state.
Here are the flame colours for common metal ions:
| Metal Ion | Flame Colour |
|---|---|
| Lithium (Li⁺) | Crimson Red |
| Sodium (Na⁺) | Yellow |
| Potassium (K⁺) | Lilac |
| Calcium (Ca²⁺) | Orange-Red |
| Barium (Ba²⁺) | Green |
| Copper(II) (Cu²⁺) | Blue-Green |
Using Sodium Hydroxide
Another common method for identifying metal ions is by mixing the solution containing the metal ions with sodium hydroxide (NaOH). When metal ions react with sodium hydroxide, they often form insoluble precipitates.
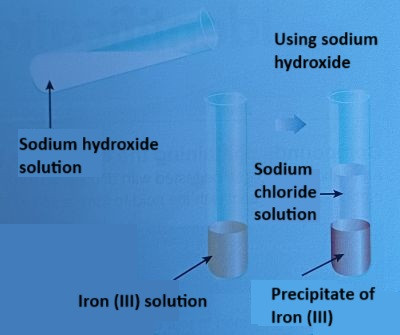
The Test:
- Add a few drops of sodium hydroxide solution to a sample of the metal ion solution.
- Observe the formation of a precipitate, which is an insoluble solid that forms when a metal ion reacts with a hydroxide ion (OH⁻).
The formation of different colour precipitates helps to identify the metal ion in the solution.
The Precipitates Formed When Metal Ions are Mixed with Sodium Hydroxide Solution
Here is a table showing the metal ions, the precipitates that form when they react with sodium hydroxide, and their characteristic colours:
| Metal Ion | Precipitate | Precipitate Colour | Equation for the Formation of Precipitate |
|---|---|---|---|
| Copper(II) (Cu²⁺) | Copper(II) hydroxide | Blue | $\text{Cu}^{2+} (aq) + 2\text{OH}^-(aq) \rightarrow \text{Cu(OH)}_2(s)$ |
| Iron(II) (Fe²⁺) | Iron(II) hydroxide | Green | $\text{Fe}^{2+} (aq) + 2\text{OH}^-(aq) \rightarrow \text{Fe(OH)}_2(s)$ |
| Iron(III) (Fe³⁺) | Iron(III) hydroxide | Brown | $\text{Fe}^{3+} (aq) + 3\text{OH}^-(aq) \rightarrow \text{Fe(OH)}_3(s)$ |
| Calcium (Ca²⁺) | Calcium hydroxide | White | $\text{Ca}^{2+} (aq) + 2\text{OH}^-(aq) \rightarrow \text{Ca(OH)}_2(s)$ |
| Magnesium (Mg²⁺) | Magnesium hydroxide | White | $\text{Mg}^{2+} (aq) + 2\text{OH}^-(aq) \rightarrow \text{Mg(OH)}_2(s)$ |
| Aluminium (Al³⁺) | Aluminium hydroxide | White (dissolves in excess NaOH) | $\text{Al}^{3+} (aq) + 3\text{OH}^-(aq) \rightarrow \text{Al(OH)}_3(s)$ |
| Zinc (Zn²⁺) | Zinc hydroxide | White (dissolves in excess NaOH) | $\text{Zn}^{2+} (aq) + 2\text{OH}^-(aq) \rightarrow \text{Zn(OH)}_2(s)$ |
Additional Notes:
- Calcium: The precipitate formed is white and is often slightly soluble in water.
- Magnesium: The precipitate is also white, and the substance is only slightly soluble in water.
- Aluminium and Zinc: Both form a white precipitate initially, but when excess sodium hydroxide is added, they dissolve to form colourless solutions. This is a unique feature that distinguishes these metals from others.
Summary of Key Reactions
- Flame tests give a quick indication of the presence of certain metal ions based on the colour of the flame produced.
- Sodium hydroxide tests help identify metal ions based on the colour of the precipitate formed when the metal ions react with hydroxide ions.
Summary Table of Flame and Precipitation Tests
| Metal Ion | Flame Colour | Precipitate with NaOH | Precipitate Colour |
|---|---|---|---|
| Lithium (Li⁺) | Crimson Red | - | - |
| Sodium (Na⁺) | Yellow | - | - |
| Potassium (K⁺) | Lilac | - | - |
| Calcium (Ca²⁺) | Orange-Red | Calcium hydroxide | White |
| Barium (Ba²⁺) | Green | - | - |
| Copper(II) (Cu²⁺) | Blue-Green | Copper(II) hydroxide | Blue |
| Iron(II) (Fe²⁺) | - | Iron(II) hydroxide | Green |
| Iron(III) (Fe³⁺) | - | Iron(III) hydroxide | Brown |
| Magnesium (Mg²⁺) | - | Magnesium hydroxide | White |
| Aluminium (Al³⁺) | - | Aluminium hydroxide | White (dissolves in excess NaOH) |
| Zinc (Zn²⁺) | - | Zinc hydroxide | White (dissolves in excess NaOH) |
Identifying metal ions is crucial for chemical analysis and can be achieved using simple techniques like flame tests and precipitation reactions with sodium hydroxide. By observing the flame colours or the colour of the precipitate formed, you can quickly identify which metal ion is present in a solution.
