Identification of Gases
In this section, we will explore how to identify the presence of different gases. Knowing how to test for gases is an essential part of chemical analysis, as it helps chemists to determine the composition of substances in various reactions.
Testing for Hydrogen
Hydrogen is a colourless, odourless, and highly flammable gas. A common test for hydrogen is the pop test.
The Test:
- Take a small amount of the gas in a test tube or container.
- Hold a lit splint at the opening of the test tube.
- If hydrogen is present, you will hear a characteristic "pop" sound.

Explanation:
The "pop" sound occurs because hydrogen reacts explosively with oxygen in the air to form water, releasing energy in the form of sound. This is a rapid combustion reaction.
Testing for Oxygen
Oxygen is essential for combustion and supports the burning of substances. A simple test to identify oxygen is the glowing splint test.
The Test:
- Take a glowing splint (a splint that has been ignited and then blown out, leaving it smouldering but not fully alight).
- Place the glowing splint into the gas you want to test.
- If oxygen is present, the splint will relight, indicating that oxygen has supported the combustion of the splint.

Explanation:
Oxygen is required for combustion to continue. When a glowing splint is placed in oxygen, it reacts with the splint, reigniting it due to the oxygen supporting the combustion.
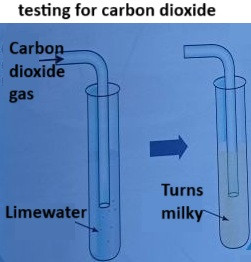
Testing for Carbon Dioxide
Carbon dioxide (CO₂) is a colourless, odourless gas that is produced during respiration and combustion. One of the most common tests for carbon dioxide is using limewater.
The Test:
- Bubble the gas through limewater (a solution of calcium hydroxide, Ca(OH)₂).
- If carbon dioxide is present, the limewater will turn from colourless to milky or cloudy.
Explanation:
Carbon dioxide reacts with calcium hydroxide in limewater to form calcium carbonate, which is insoluble in water and causes the solution to turn milky.
$$\text{Ca(OH)}_2 (aq) + \text{CO}_2 (g) \rightarrow \text{CaCO}_3 (s) + \text{H}_2\text{O} (l)$$
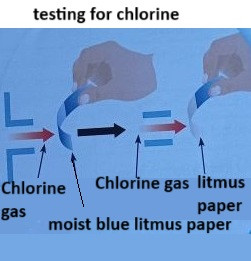
Testing for Chlorine
Chlorine is a greenish-yellow, toxic gas with a pungent odour. It is often produced in industrial processes and can be tested for using damp litmus paper.
The Test:
- Take a piece of damp litmus paper (either red or blue).
- Place the litmus paper near the gas or expose it to the gas in a test tube.
- If chlorine is present, the litmus paper will first turn red due to the acidic nature of chlorine, and then it will be bleached (turning colourless).
Explanation:
Chlorine is acidic and reacts with the litmus paper to form hydrochloric acid and hypochlorous acid. These acids cause the red litmus paper to turn colourless due to the bleaching effect.
Summary of Gas Tests
| Gas | Test Method | Observation |
|---|---|---|
| Hydrogen | Place a lit splint at the opening of a test tube of the gas | "Pop" sound |
| Oxygen | Place a glowing splint in the gas | Splint reignites |
| Carbon Dioxide | Bubble the gas through limewater | Limewater turns milky |
| Chlorine | Expose damp litmus paper to the gas | Litmus paper turns red then bleaches (colourless) |
Testing for gases is a key part of chemical analysis. Each gas has its own unique way of reacting in a test, allowing chemists to identify it with confidence. Understanding these tests will help you to identify gases in laboratory experiments or real-world applications, such as monitoring gases in industrial processes or environmental science.
