Collision Theory, Activation Energy, and Catalysts
In this section, we will explore the factors that affect the rate of a chemical reaction, focusing on Collision Theory, Activation Energy, and the role of Catalysts. These concepts are essential for understanding how and why reactions occur at different rates.
Collision Theory
Collision Theory helps us understand how and why chemical reactions happen. It states that for a reaction to take place, the reacting particles must collide with each other. However, not all collisions result in a reaction. For a collision to be successful, it must meet two key conditions:
Sufficient Energy: The particles must collide with enough energy to overcome the energy barrier (activation energy) needed for the reaction to occur.
Correct Orientation: The particles must collide in the right orientation, allowing the bonds to break and new bonds to form, leading to the creation of products.
The rate of reaction is determined by how often particles collide and how effectively these collisions lead to the formation of products.
Activation Energy
Activation Energy is the minimum amount of energy that particles must have in order to react. Even if particles collide, if they do not possess sufficient activation energy, they will simply bounce off each other without reacting.
The higher the activation energy, the fewer particles in the reaction mixture will have enough energy to react at a given temperature, which means the reaction will be slower. Conversely, if the activation energy is low, more particles will have enough energy to react, and the reaction will occur more quickly.
Factors Affecting the Rates of Reaction
Several factors can influence how often particles collide and how successful those collisions are. These include:
Surface Area
The surface area of a solid reactant is crucial in determining the rate of reaction. The larger the surface area, the more particles are exposed to the other reactant(s), leading to more frequent collisions.
- For example: If a large piece of a solid reactant is used, only the surface can react with the other substance. However, if the solid is broken into smaller pieces or powdered, there is a greater surface area, meaning more particles are available to collide with the reactant, increasing the rate of reaction.
Temperature
Temperature has a significant effect on the rate of reaction. When the temperature is increased, the particles move faster because they have more kinetic energy. This leads to more frequent collisions, and a greater proportion of these collisions will have enough energy to overcome the activation energy barrier.
- For example: If a reaction is heated, the molecules will collide more often and with greater energy, causing the reaction to occur more quickly. Conversely, lowering the temperature slows the particles down, reducing the rate of reaction.
Pressure (for Gaseous Reactions)
Pressure is relevant for reactions involving gases. Increasing the pressure of a gas causes the particles to become more tightly packed, which leads to more frequent collisions.
- For example: If the pressure is increased in a gaseous reaction, the particles are pushed closer together, increasing the likelihood of collisions and speeding up the reaction rate. This principle is important in industrial processes, such as the Haber process for ammonia production.
Concentration (for Solutions)
For reactions that involve solutions, the concentration of reactants plays an important role. The higher the concentration of reactants, the more particles there are in a given volume, meaning collisions are more likely to occur.
- For example: If a solution is more concentrated, there are more reactant particles in the same volume, which increases the frequency of collisions and speeds up the rate of reaction. Conversely, if the concentration is lower, there are fewer particles to collide, and the reaction rate decreases.
Catalysts and How They Change a Chemical Reaction
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysts work by providing an alternative reaction pathway with a lower activation energy. This allows more particles to have enough energy to react, thereby increasing the rate of reaction.
- How Catalysts Work: Catalysts provide a surface for reactant particles to adsorb onto, increasing the number of successful collisions. By lowering the activation energy, catalysts make it easier for the reactants to form products. Importantly, catalysts are not used up in the reaction and can be reused.
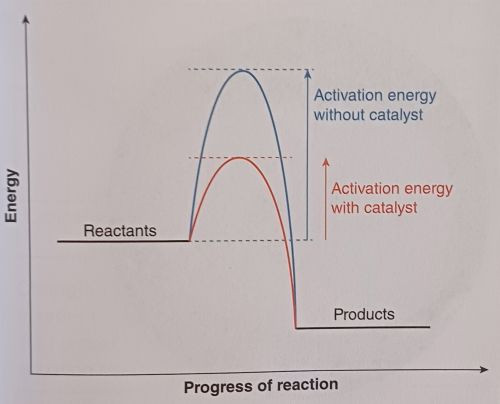
Examples of Catalysts:
- In industry: In the manufacture of ammonia using the Haber process, iron is used as a catalyst.
- In biology: Enzymes are biological catalysts that speed up reactions in living organisms. For example, the enzyme catalase breaks down hydrogen peroxide into water and oxygen in the body.
Summary
In summary, the rate of a chemical reaction is influenced by several factors, including the surface area, temperature, pressure, and concentration of the reactants. Catalysts are substances that speed up reactions by lowering the activation energy required for the reaction to occur. By understanding these concepts, we can control and optimise reactions in both laboratory and industrial settings. By using Collision Theory, we can explain why reactions happen faster under certain conditions, and by adjusting the factors affecting the rate, we can control how quickly reactions proceed.
