Rates of Reaction
In this section, we will explore how fast chemical reactions occur and the factors that influence the rate at which they take place. Understanding the rate of a reaction is crucial, as it helps us control processes in industries such as pharmaceuticals, food production, and manufacturing.
Calculating the Rates of Reaction
The rate of a reaction refers to how quickly the reactants are converted into products. There are several ways to measure this rate, depending on the type of reaction. The rate can be calculated using the following formula:
$$\text{Rate of reaction} = \frac{\text{Change in concentration of reactant or product}}{\text{Time taken}}$$
For example:
- If you are measuring how fast a reactant is being used up, you would measure the decrease in the concentration of that reactant over time.
- If you are measuring how fast a product is being formed, you would measure the increase in the concentration of the product over time.
The units for rate of reaction depend on what is being measured, but typically they are expressed in units like mol/s, g/s, or cm³/s.
Mean Rate of Reaction
The mean rate of reaction can be calculated by using the total change in concentration of a reactant or product over the total time taken for the reaction. It can be calculated using the formula:
$$\text{Mean rate of reaction} = \frac{\text{Total change in concentration}}{\text{Total time taken}}$$
This provides an average rate over the entire course of the reaction. However, if the reaction rate changes during the reaction, it is often more useful to calculate the rate at a particular time using instantaneous rates (measuring how the rate changes at specific points).
Factors Affecting the Rates of Reaction
Several factors can affect how quickly a chemical reaction occurs. These include:
Concentration of reactants: A higher concentration of reactants leads to more frequent collisions between particles, which increases the rate of reaction.
Temperature: Increasing the temperature increases the energy of the particles, causing them to collide more frequently and with greater energy. This typically speeds up the reaction.
Surface area: A larger surface area (such as smaller pieces of solid reactants) increases the rate of reaction because there is more area for collisions to occur.
Catalysts: A catalyst is a substance that increases the rate of a reaction without being used up in the process. It provides an alternative pathway with lower activation energy for the reaction.
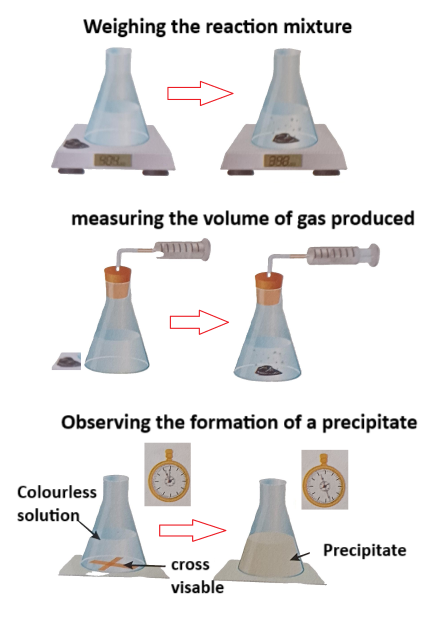
Weighing the Reaction Mixture
One common method for measuring the rate of a reaction involves weighing the reaction mixture. This is particularly useful when a gas is produced. By monitoring the mass of the system over time, you can calculate how fast the reactants are being converted into products.
For example, in a reaction where a gas is released (e.g., the reaction between marble chips and hydrochloric acid), the loss of mass can be tracked. The rate of reaction can be calculated by measuring how much mass is lost over a specific time period.
Measuring the Volume of Gas Produced
For reactions where gases are produced, one method of measuring the rate of reaction is by collecting and measuring the volume of gas produced at different time intervals. This can be done using a gas syringe or an inverted measuring cylinder in a water bath.
To calculate the rate of reaction using this method, the volume of gas produced is measured at regular time intervals. The rate is then determined by calculating the change in volume over time. This is often used in reactions such as the reaction between hydrochloric acid and zinc, where hydrogen gas is produced.
Observing the Formation of a Precipitate
Another method of measuring the rate of a reaction is by observing the formation of a precipitate. A precipitate is a solid that forms when two solutions react. In some reactions, the formation of a precipitate can be used to monitor the rate at which the reaction occurs.
For example, when sodium thiosulphate reacts with hydrochloric acid, a yellow precipitate of sulphur forms, making the solution cloudy. By timing how long it takes for the solution to become opaque, you can calculate the rate of the reaction. The faster the precipitate forms, the faster the reaction is taking place.
Summary
In summary, the rate of a chemical reaction can be measured in different ways, including by monitoring changes in concentration, measuring the volume of gas produced, or observing the formation of a precipitate. The rate of reaction can be influenced by factors such as concentration, temperature, surface area, and the presence of a catalyst. By understanding these factors and methods, we can better control chemical reactions in a range of scientific and industrial processes.
