Alternative Methods for Extracting Metals
This section highlights alternative methods of extracting metals covering extracting copper, phytomining, bioleaching, processing metal compounds, processing metal compounds and provides a summary of the methods used to extract metals.
Extracting Copper
Copper is a valuable metal used in electrical wiring, plumbing, and in various alloys. Traditionally, copper has been extracted from its ores, such as copper sulphide (CuS) or copper oxide (CuO), through smelting, where the ore is heated with carbon. This process is energy-intensive and releases harmful gases into the atmosphere. However, there are alternative, more sustainable methods to extract copper, which are particularly important as the supply of high-grade ores decreases.
Phytomining
Phytomining is an innovative technique used to extract metals, particularly copper, from low-grade ores or contaminated soil. It involves growing specific plants that are able to absorb metal ions from the soil through their roots. These plants are known as hyperaccumulators, and they can take up copper and other metals into their tissues as they grow.
Process: Once the plants have grown, they are harvested and then burnt to produce ash. The ash contains concentrated metal compounds, which can be processed to extract the metal. In the case of copper, the copper compounds in the ash can be extracted using chemical methods.
Advantages:
- Environmentally friendly: Phytomining reduces the need for traditional mining, which can cause significant environmental damage such as habitat destruction and soil erosion.
- Cost-effective: It can be more economical than extracting copper from low-grade ores using conventional methods.
- Rehabilitation of contaminated land: Phytomining can also help to clean up land contaminated by heavy metals, as the plants absorb toxic substances.
Disadvantages:
- Slow process: Growing the plants and harvesting them takes time, so phytomining is slower compared to traditional mining methods.
- Limited to specific locations: The technique is only suitable for areas where the soil contains metal compounds and where the plants can grow effectively.
Bioleaching
Bioleaching is another environmentally friendly technique used to extract metals, including copper, from low-grade ores or waste materials. It involves using bacteria or other microorganisms to break down the ore and release metal ions into a solution.
Process: Certain bacteria, such as Acidithiobacillus ferrooxidans, can oxidise the metals in the ore, turning them into soluble metal ions. These ions are then leached out of the ore and collected in solution, where they can be processed further to extract the metal.
Advantages:
- Less environmental damage: Unlike traditional mining methods, bioleaching does not require large amounts of energy or cause destruction to the landscape.
- Suitable for low-grade ores: It allows for the extraction of metals from ores that are not worth processing by conventional methods.
Disadvantages:
- Slow process: Bioleaching can take a long time to extract the desired metal, and the process needs to be carefully managed to ensure the microorganisms remain active.
- Possible contamination: If not managed correctly, the bacteria can produce harmful substances, which may lead to environmental pollution.
Processing Metal Compounds
Once metals have been extracted from ores or obtained through alternative methods like phytomining or bioleaching, they must be purified or refined. Two common methods for processing metal compounds to extract pure metals are displacement reactions and electrolysis.
Displacement Reactions
A displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. In metal extraction, this is often used to obtain metals from metal compounds.
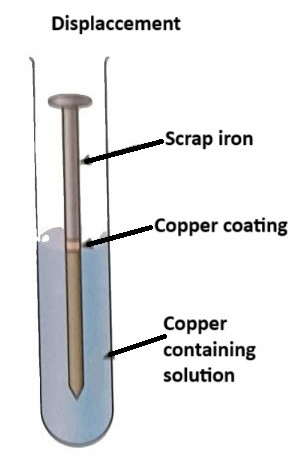
For example:
Process: A metal, such as zinc, is used to displace copper from a copper salt solution (copper sulphate solution):
$$\text{Zn} (s) + \text{CuSO₄} (aq) → \text{ZnSO₄} (aq) + \text{Cu} (s)$$
In this reaction, zinc is more reactive than copper, so it displaces copper from the solution, leaving pure copper metal behind.
Advantages: Displacement reactions are relatively simple and inexpensive, and they do not require complex equipment.
Disadvantages: The method is limited to situations where a more reactive metal can displace the target metal. It is not suitable for extracting all metals from their compounds.
Electrolysis
Electrolysis is a more complex and energy-intensive process used to extract metals from their compounds, especially for metals that are more reactive, such as aluminium and sodium. Electrolysis involves passing an electric current through a molten compound or solution, causing the metal ions to move to the electrodes where they are reduced to form pure metal.
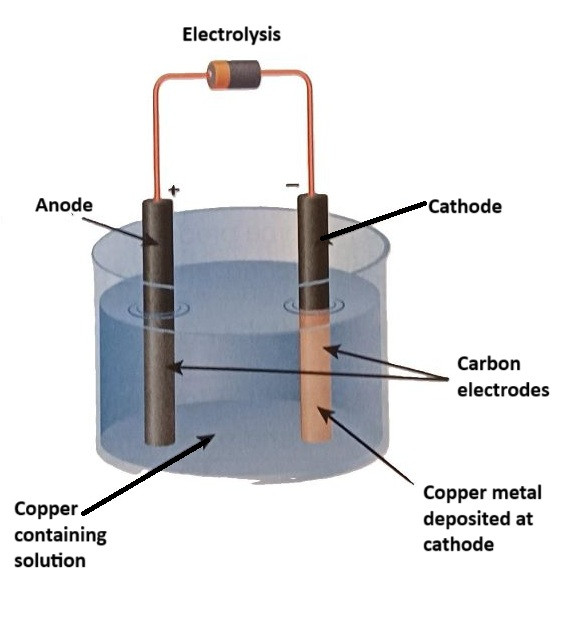
Process: In the case of copper extraction, copper is typically purified using electrolysis. Copper sulphate solution is used as the electrolyte, and an electric current is passed through the solution. At the cathode (the negative electrode), copper ions gain electrons and are deposited as pure copper:
$$\text{Cu}^{2+} (aq) + 2e⁻ → \text{Cu} (s)$$
At the anode (the positive electrode), copper from the impure copper electrode dissolves to release copper ions into the solution:
$$\text{Cu} (s) → \text{Cu}^{2+} (aq) + 2e⁻$$
Advantages: Electrolysis can produce very pure metals, which are essential for certain applications (e.g., in electronics and electrical wiring).
Disadvantages: Electrolysis is an energy-intensive process, requiring high temperatures and significant amounts of electricity, which makes it expensive. The process is not suitable for all types of metal extraction.
Summary of Methods for Extracting Metals
| Method | Process | Advantages | Disadvantages |
|---|---|---|---|
| Phytomining | Plants absorb metal ions from soil, then harvested and processed. | Environmentally friendly, cost-effective, helps clean contaminated land. | Slow process, limited to specific locations. |
| Bioleaching | Bacteria break down ores to release metal ions into solution. | Less environmental damage, suitable for low-grade ores. | Slow process, requires careful management. |
| Displacement | A more reactive metal displaces a less reactive metal from its compound. | Simple and inexpensive. | Limited to suitable metals; cannot be used for all extractions. |
| Electrolysis | Electric current passes through molten compound or solution to extract metal. | Produces pure metal. | Energy-intensive, expensive. |
The extraction of metals from ores is an essential part of modern industry. While traditional methods like smelting are still used for certain metals, alternative methods such as phytomining, bioleaching, displacement reactions, and electrolysis are becoming increasingly important. These methods offer more environmentally friendly options and can be used to extract metals from low-grade ores or waste materials. The choice of extraction method depends on factors such as the type of metal, the ore composition, and the environmental impact of the process.
