Cracking and Alkanes
In this section, we will explore cracking and alkanes, focusing on the process of cracking long-chain hydrocarbons into shorter-chain hydrocarbons, and the characteristics and reactions of alkanes.
Long-Chain Hydrocarbons and Short-Chain Hydrocarbons
Long-chain hydrocarbons are compounds that contain a large number of carbon atoms joined together in chains, often found in crude oil. These molecules are often viscous and not ideal for use in fuels. However, the demand for shorter-chain hydrocarbons, which are more useful as fuels (e.g., petrol, diesel), has led to the process of cracking.
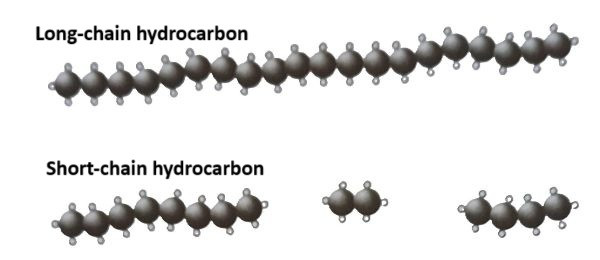
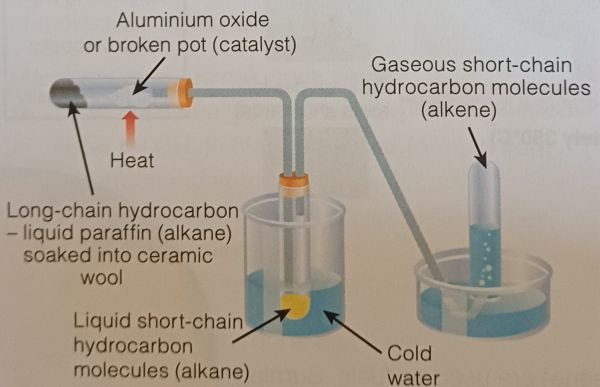
Cracking is the process of breaking down large, long-chain hydrocarbons into smaller, more useful molecules. This is typically done by applying heat and sometimes a catalyst. Short-chain hydrocarbons (e.g., methane, ethane, propane) are lighter, more volatile, and more desirable for fuel use, making them ideal for various applications such as petrol, gas for cooking, and as raw materials in the chemical industry.
Alkanes
Alkanes are saturated hydrocarbons, meaning they only contain single bonds between carbon atoms. Alkanes follow the general formula CₙH₂ₙ₊₂. They are relatively unreactive but undergo reactions such as combustion and substitution reactions with halogens. The alkanes can be found in crude oil and are typically used as fuels.
Names and Displayed Formulas of Alkanes
The following table shows the names of some common alkanes and their displayed formulas:
| Name of Alkane | Displayed Formula |
|---|---|
| Methane | CH₄ |
| Ethane | C₂H₆ |
| Propane | C₃H₈ |
| Butane | C₄H₁₀ |
| Pentane | C₅H₁₂ |
| Hexane | C₆H₁₄ |
| Heptane | C₇H₁₆ |
These alkanes are used for various purposes, with methane being a primary component of natural gas, and propane and butane used in gas bottles for heating and cooking.
Alkanes and Their Reactions
Alkanes are generally unreactive, but they can undergo specific reactions, especially with halogens (such as chlorine, bromine, and iodine). In these reactions, the alkane undergoes a substitution reaction. The general mechanism involves the halogen replacing one of the hydrogen atoms in the alkane molecule, typically in the presence of ultraviolet (UV) light.
Alkanes can also undergo combustion reactions with oxygen, producing carbon dioxide and water.
Reactions of Alkanes
Here is a table of some key reactions involving alkanes, showing the reactants, the notes, and the general equations:
| Reactant | Notes | Equation |
|---|---|---|
| Oxygen | Alkanes burn in oxygen (combustion) to produce energy. Complete combustion produces carbon dioxide and water. | $\text{C₄H₁₀} + 13\text{O₂} \rightarrow 4\text{CO₂} + 5\text{H₂O}$ |
| Hydrogen | Hydrogenation: Alkanes can be hydrogenated (adding hydrogen) in the presence of a catalyst to form larger alkanes. | $\text{C₆H₁₄} + \text{H₂} \rightarrow \text{C₈H₁₈}$ |
| Water | Alkanes react with steam to produce alcohols under high pressure and temperature. | $\text{C₆H₁₄} + \text{H₂O} \rightarrow \text{C₆H₁₄OH}$ |
| Halogens | Halogenation: Alkanes react with halogens (like chlorine) in the presence of UV light. A hydrogen atom is replaced by a halogen atom. | $\text{CH₄} + \text{Cl₂} \xrightarrow{\text{UV light}} \text{CH₃Cl} + \text{HCl}$ |
Summary
In summary, cracking is the process of breaking down long-chain hydrocarbons into shorter-chain hydrocarbons that are more useful for fuel and other applications. Alkanes are saturated hydrocarbons that follow the general formula CₙH₂ₙ₊₂, and they undergo reactions such as combustion, hydrogenation, halogenation, and reactions with water. Understanding alkanes and cracking is fundamental to understanding how fuels and other products are derived from crude oil.
