Crude Oil, Hydrocarbons, and Alkanes
In this section, we will explore crude oil, hydrocarbons, and alkanes. These are fundamental concepts in organic chemistry. Understanding them is essential for understanding how fuels and many other products are made.
Crude Oil and How It Is Formed
Crude oil is a natural, unrefined petroleum product found deep beneath the Earth’s surface. It is formed over millions of years from the remains of ancient marine organisms, such as plankton and algae. These remains are buried under layers of sand, mud, and rock. Over time, heat and pressure break down the organic material into hydrocarbons (compounds made up of hydrogen and carbon atoms).
The process of forming crude oil takes millions of years, as the organic material is subjected to increasing pressure and temperature. As a result, crude oil is a mixture of many different hydrocarbons, ranging from small molecules to large complex ones.
Hydrocarbons
A hydrocarbon is a compound made up only of carbon and hydrogen atoms. Hydrocarbons can be classified into two main types: alkanes and unsaturated hydrocarbons (such as alkenes).
- Alkanes are saturated hydrocarbons, meaning they only contain single bonds between carbon atoms. They are the simplest type of hydrocarbons and are generally unreactive, except for combustion.
- Unsaturated hydrocarbons, such as alkenes, contain at least one double bond between carbon atoms. They are more reactive and are used in various chemical processes, including polymerisation.
Alkanes
Alkanes are a group of hydrocarbons that contain only single bonds between carbon atoms. The general formula for alkanes is CₙH₂ₙ₊₂. This means that for every carbon atom, there are two hydrogen atoms for each bond, plus two extra hydrogen atoms for the entire molecule.
Alkanes are saturated hydrocarbons, which means they contain the maximum number of hydrogen atoms possible for the number of carbon atoms present. The simplest alkane is methane (CH₄), which consists of one carbon atom bonded to four hydrogen atoms. As the number of carbon atoms increases, the alkanes become more complex, such as ethane (C₂H₆), propane (C₃H₈), and butane (C₄H₁₀).
Alkanes are generally unreactive, but they can undergo combustion (burning) and react with halogens under certain conditions.
Fractional Distillation
Fractional distillation is the process used to separate the different components of crude oil based on their boiling points. Crude oil is a complex mixture of hydrocarbons with a range of boiling points, and fractional distillation allows us to separate these components into different fractions.
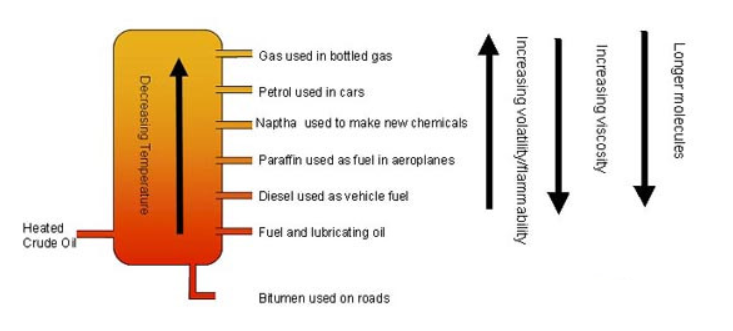
The process works as follows:
- Crude oil is heated in a furnace until it vaporises.
- The vapour enters a fractionating column, which is cooler at the top and hotter at the bottom.
- As the vapour rises up the column, the hydrocarbons cool and condense at different heights, where the temperature corresponds to their boiling points.
- The hydrocarbons with higher boiling points condense lower down the column, while those with lower boiling points condense higher up.
The fractions collected include gases like methane (used in cooking and heating), petrol (used in cars), kerosene (used as jet fuel), and diesel oil. The fraction with the highest boiling point is used for bitumen, which is used in road construction.
Combustion of Hydrocarbons
The combustion of hydrocarbons is a chemical reaction where a hydrocarbon reacts with oxygen to produce carbon dioxide and water. The general equation for the combustion of a hydrocarbon is:
$$\text{Hydrocarbon} + \text{Oxygen} \rightarrow \text{Carbon Dioxide} + \text{Water}$$
For example, the combustion of methane (CH₄), the simplest alkane, is represented by the following equation:
$$\text{CH₄} + 2\text{O₂} \rightarrow \text{CO₂} + 2\text{H₂O}$$
There are two types of combustion:
Complete combustion occurs when there is a sufficient supply of oxygen, producing carbon dioxide (CO₂) and water (H₂O) as the only products.
Example: When methane burns in a plentiful supply of oxygen:
$$\text{CH₄} + 2\text{O₂} \rightarrow \text{CO₂} + 2\text{H₂O}$$
Incomplete combustion occurs when there is a limited supply of oxygen, which leads to the production of carbon monoxide (CO), soot (carbon), or both, along with water. This is less efficient and can be dangerous due to the production of toxic carbon monoxide.
Example: In incomplete combustion, the equation for methane might look like this:
$$\text{2CH₄} + 3\text{O₂} \rightarrow 2\text{CO} + 4\text{H₂O}$$
In this case, carbon monoxide (CO) is produced instead of carbon dioxide (CO₂).
Summary
In summary, crude oil is a natural resource that is formed over millions of years from the remains of ancient marine organisms. It is a mixture of hydrocarbons, which are compounds made of carbon and hydrogen. Alkanes are saturated hydrocarbons with single bonds between carbon atoms, and they are the simplest type of hydrocarbons. Fractional distillation is used to separate crude oil into its components based on boiling points, and combustion of hydrocarbons produces carbon dioxide and water, with complete combustion producing only these products, and incomplete combustion leading to the formation of carbon monoxide and soot. Understanding these concepts is essential for understanding how fossil fuels are used in energy production and other industrial processes.
