Electrolysis of Brine
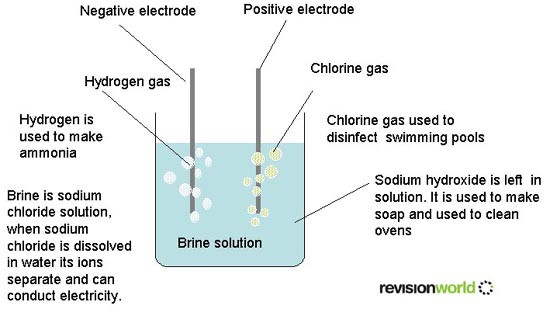
Brine is a solution of sodium chloride (NaCl) and water (H2O). The process of electrolysis involves using an electric current to bring about a chemical change and make new chemicals. The electrolysis of brine is a large-scale process used to manufacture chlorine from salt. Two other useful chemicals are obtained during the process, sodium hydroxide (NaOH) and hydrogen (H2).
It is important that the chlorine and sodium hydroxide produced in the process are separated they react when they come into contact with each other.
Uses in industry
Product of electrolysis of brine
- Chlorine - disinfectant and purifier, manufacture of hydrochloric acid and making plastics
- Sodium Hydroxide - processing food products, removing pollutants from water and manufacture of paper
- Hydrogen - manufacture of hydrochloric acid and potential as a pollution-free fuel
Environmental impacts of the electrolysis of brine on a large scale must be considered. The process uses a lot of electricity that is mainly produced by the burning of fossil fuels. During the actual process of electrolysis, metal must be in contact with the solution of brine. A metal commonly used is mercury which is toxic. Some mercury escapes into the solution and into the environment.
This video explain more about Electrolysis
