Bonding
Compounds are substances in which atoms of two, or more, elements are not just mixed together but chemically combined. This is known as Bonding.
Chemical reactions between elements involve either the giving and taking, or sharing, of electrons in the highest occupied energy levels of atoms.
This video explains about Bonding and the different types of bonding
Covalent Bonding
When non-metals react together both atoms need to gain electrons to obtain a full shell of electrons.
The atoms are held together by shared pairs of electrons.
The shared pair of electrons is a covalent bond.
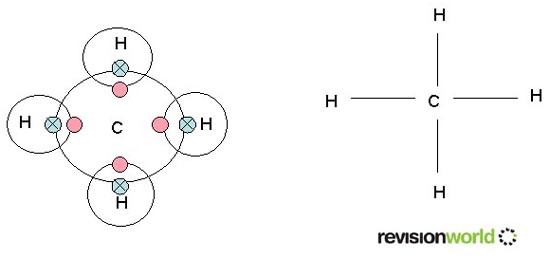
Methane is formed from one carbon atom and four hydrogen atoms.
Each carbon has four outer electrons and each hydrogen atom has 1 outer electron.
Sharing gives both types of atom full outer shells.
Ionic Bonding
Atoms lose or gain electrons to attain a complete outer shell of electrons.
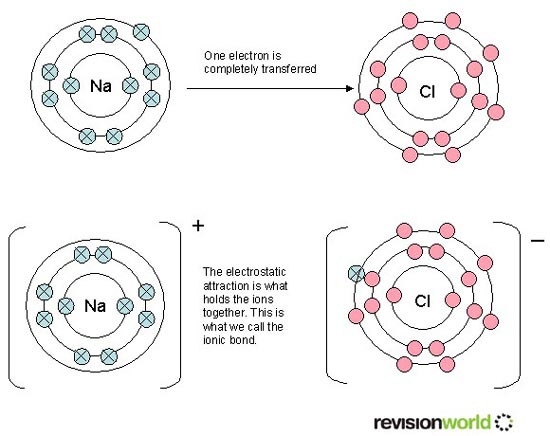
An ionic bond is formed when electrons are lost and gained by two or more atoms.
When atoms lose electrons they become positive ions
When atoms gain electrons they become negative ions
Ionic bonds are formed between metals and non - metals.
Metallic Bonding
In metals, positive metal ions are held together by electron clouds. This is known as metallic bonding.
These electrons are free to move through the structure, this is why metals conduct electricity.
This can explain the change in melting points as you go down group I.
The melting points decrease as you go down the group. The atoms get larger as you go down the group (because they have more shells of electrons).
The group I metals all have one electron in the outside shell. Think of this as the glue that holds the metal structure together.
If we have small atoms and a certain amount of glue, we will have fairly big attractions between the atoms and so the melting point will be quite high.
If we have large atoms and the same amount of glue (still one electron available from the outside shell) there will be less attraction and so the melting point will be lower.
This can also help to explain why the group I metals are soft. Hardness depends on the attraction between the atoms. Lots of attraction means the metal will be hard. None of the group I metals can be described as “hard” but as you go down the group, they get even softer.
