Useful Products from Air
This sectiom explains Useful Products from the Air.
Manufacture of Ammonia from the Air
- Nitrogen and Hydrogen are needed to make Ammonia.
- Nitrogen is obtained from the air.
- Hydrogen is obtained from water and natural gas.
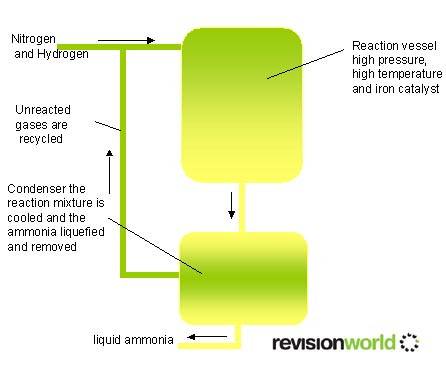
- The Haber process is a reversible reaction
- This means that the reaction occurs in both directions
- High pressures favour the production of ammonia, however it expensive to make industrial equipment to cope with high pressures.
- Low temperatures favour the production of ammonia, however at low temperatures the reaction would be too slow to be commercially viable.
- The Haber process makes a compromise with these two and recycles the unreacted hydrogen and nitrogen
- N2(g) + 3H2(g) <> 2NH3(g) . <> menas the reaction is reversible
Manufacture of Fertiliser from Ammonia
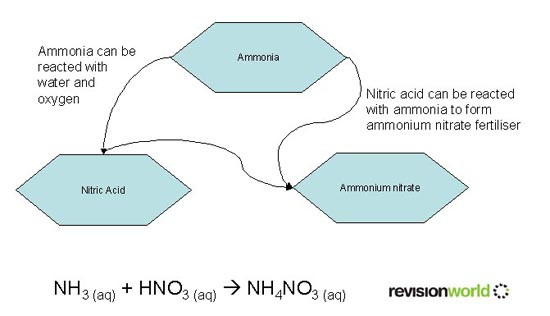
This video overviews the use of Ammonia and Fertilisers
Overuse of Fertilsers and the problems
Plants need several types of nutrients.
Nitrogen based nutrients are used to make proteins. A plant grown in soil with too little nitrogen will be weak.
Farmers can use natural sources of these nutrients or synthetic nutrients. Natural sources include animal manure and "guano" a term meaning bird droppings. Before the success of the Haber-Bosch process, guano was imported from South America. The coming of the First World War meant that the journey across the Atlantic was too risky and yet it was vital that nitrates were still available for fertiliser and explosives.
Many historians believe that the Frist World War really would have been over much sooner if it hadn't been for the success of the Haber-Bosch process. Although the production of fertiliser is of much greater importance today, it was the need for explosives for the war that encouraged investment in the research to make the process effective.
It is not only nitrogen that is required. Phosphorus and potassium are also possibly missing from the soil needed for healthy plant growth.
Commercial fertilisers are mixtures of nitrogen compounds, phosphorus compounds and potassium compounds. The label shows the "NPK" values. These are the percentages of nitrogen, phosphorus and potassium.
If fertilisers are overused the excess can wash into streams. This can be the direct dissolving of the fertilsier in rain water that is washed off the surface of the soil or it may be a slower but very effective leaching of the chemicals through the soil. Plants and green algae grow out of control. When they die, bacteria feed off of the dead plant material The bacteria increase in number, they use up all the oxygen in the water. Then the fish die. This process is called eutrophication.
Too high a concentration of nitrates in drinking water can also cause problems. It can interfere with the blood’s ability to carry oxygen. This can be especially severe in children and babies, causing them to turn blue and even die. Stomach cancer has also been linked to high nitrate levels.
Although it is difficult to set a precise value for the safe level of nitrate in drinking water, everyone agrees that the less nitrates there are, the better.
