The Alkali Metals: Group 1 of the Periodic Table
Group 1 of the Periodic Table
In group 1 of the periodic table you will find the Alkali Metals.
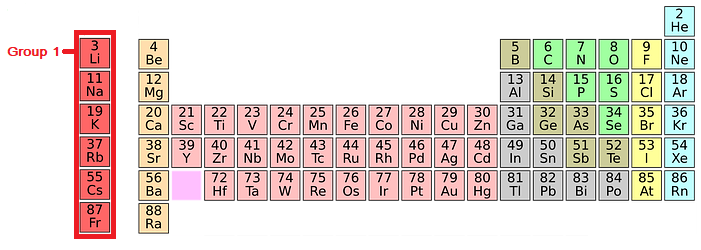
Alkali metals have the following properties:
- They have a low density (lithium, sodium and potassium float on water).
- They form ionic compounds in which the metal ion has a charge of + 1 by reacting with non-metals .
- They form white solid compounds that dissolve in water to form colourless solutions.
The video below shows how Alkali Metals lithium (Li), sodium (Na) and potassium (K) react with Water.
The alkali metals react with water forming metal hydroxides which dissolve in water to form alkaline solutions and hydrogen gas. For example, for the reaction between sodium and water:
2Na + 2H2 → 2NaOH + H2
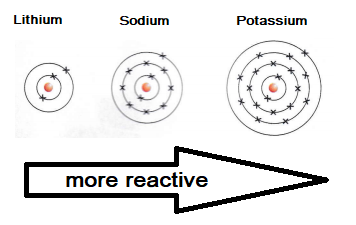
Alkali metals become more reactive as you go down the group in the periodic table. This is because the outer shell gets further away from the positive attraction of the nucleus. This makes it easier for an atom to lose an electron from its outer shell.
This video below explains more about alkali metals or group 1 metals on the periodic table.
