Atomic Structure
Scientific models of the atom
The scientific community originally believed that atoms were tiny spheres that could not be divided.
In the early 19th century John Dalton conducted experiments and concluded:
- All matter is made of indestructible atoms.
- Atoms of a particular element are identical.
- Atoms are rearranged during chemical reactions.
- Compounds are formed when two or more different types of atom join together.
Upon J. J. Thomson’s discovery of the electron in 1897, the ‘plum pudding’ model suggested that the atom was a ball of positive charge with negative electrons embedded throughout.
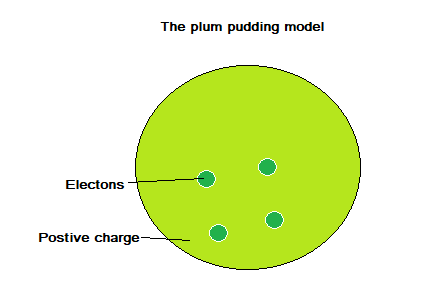
The plum pudding model was replaced by the nuclear model, following Rutherford, Geiger and Marsden’s alpha scattering experiments (conducted between 1911 and 1913).
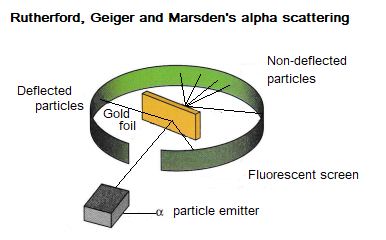
In this experiment, alpha particles (which are positive) are fired at a thin piece of gold. A few of the alpha particles are deflected as they don’t pass through the gold. This observation led Rutherford, Geiger and Marsden to suggest that this is because the positive charge of the atom is confined in a small volume (now called the nucleus).
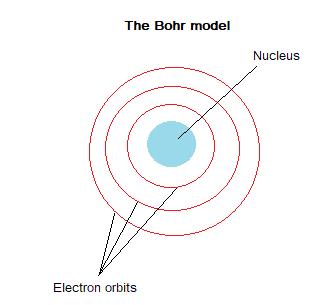
The Nuclear model was adapted by Niels Bohr in 1913, Bohr suggested that electrons orbit the nucleus at specific distances. Bohr’s theoretical calculations were backed up with experimental results. Later experiments confirmed that the positive charge of the nucleus was subdivided into smaller particles (these are now called protons), with each particle having the same amount of positive charge.
This video describes the Bohr model.
James Chadwick’s work in 1932 suggested that the nucleus also contained neutral particles (these are now called neutrons).
