Electronic Structure and the Periodic Table
Electronic structures
Electrons in an atom occupy the lowest available energy level (shell). The first energy level (closest to the nucleus) can hold up to two electrons. The second and third energy levels can hold up to eight electrons.
For example Aluminium has the atomic number of 13. This means that there are 13 protons in the nucleus of an aluminium atom and therefore there must be 13 electrons (so that the atom is neutral).
The electronic structure of Aluminium can be written as 2, 8, 3, or shown in a diagram like the one below.
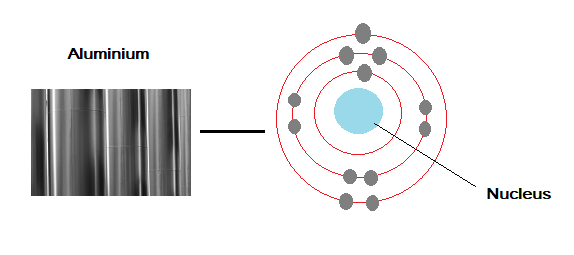
Aluminium is in group 3 of the periodic table. This is because it has three electrons in its outer shell. The chemical properties (reactions) of an element are related to the number of electrons in the outer shell of the atom.
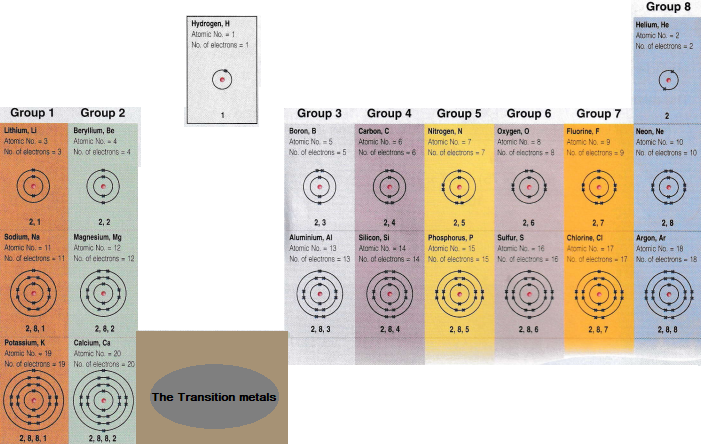
The electronic structure of the first twenty elements, are shown below.
Glossary of terms:
Energy level: A region in an atom where electrons are found.
Shell: Another word for an energy level.
