The Noble Gases: Group 0 of the Periodic Table
The Periodic Table Group 0
The elements that comprise group 0 of the periodic table are called noble gases.
Image
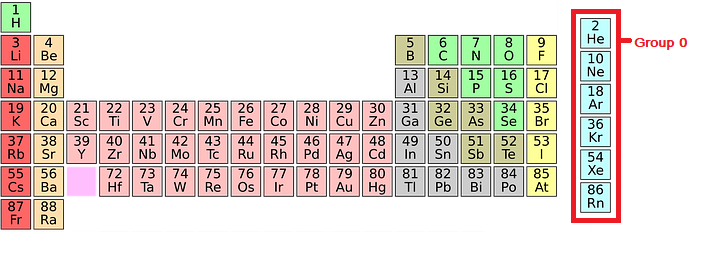
The noble gases are chemically inert (unreactive) and do not easily form molecules because their atoms have full outer shells of electrons (energy levels). Their properties make noble gases ideal for balloons, light bulbs, lasers, advertising signs and airships. This is because they are inert, have a low density and are not flammable.
Image
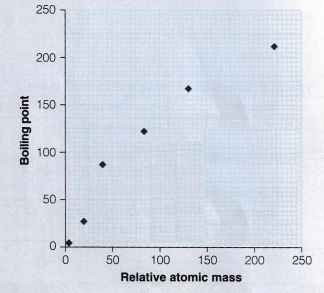
The graph above shows us that the boiling points of noble gases increases with increasing relative atomic mass, i.e., as you go down the group.
The video below provides an overview of the noble gases.
