Separating Mixtures
Chemical reactions are the only way to separate compounds. Mixtures however can be separated by physical processes. These include: Filtration, Crystallisation, Distillation and Chromatography.
How different mixtures can be separated
Filtration
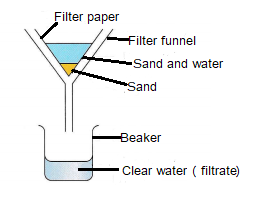
Filtration is used for separating insoluble solids from liquids, e.g. sand and water.
Crystallisation
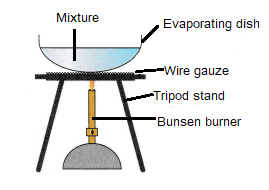
Crystallisation is used to separate solids from solutions, e.g. obtaining salt from salty water.
Simple distillation
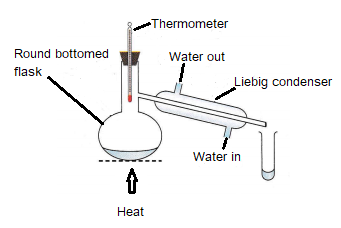
Simple distillation is used to separate a liquid from a solution, e.g. water from salty water.
Fractional distillation
Fractional distillation is used to separate liquids that have different boiling points, e.g. ethanol and water.
Chromatography
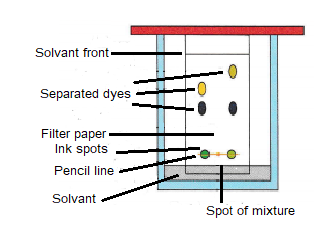
Chromatography used to separate dyes, e.g. the different components of ink.
The video below explains how mixtures can be separated.
