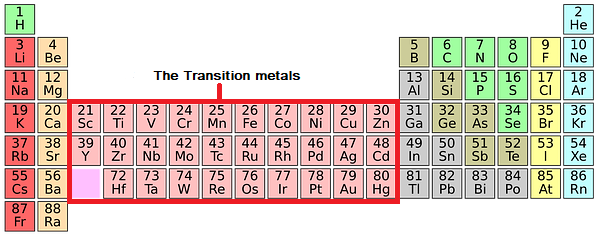
The Transition Metals
The Transition Metals
The transition metals are: Chromium, Manganese, Iron, Cobalt, Nickel and Copper.
Image
How do transition metals compare to alkali metals (group 1 metals)?
Transition metals:
- Have higher melting points (with the exception of mercury).
- Are more dense.
- Are less reactive with water and oxygen.
Properties of transition metals
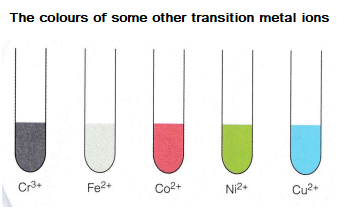
Many transition elements have ions with different charges. For example, FeCl2 is a compound that contains FE2+ ions and FeCl3 is a compound that contains Fe3+ ions.
Compounds of transition metals are also often coloured. For example, potassium manganate (VII), KMnO4, is purple and copper (II) sulphate, CuSo4, is blue.
Image

Image
Transition metals are also frequency used as catalysts, such as iron in the Haber process and nickel used in the manufacture of margarine.
The video below explains more about transition metals.
