Quantitative Chemistry Overview
Calculating Masses

Mass Number - The total number of protons and neutrons in an atom.
Relative Atomic Mass - The average mass of the atoms in a sample compared to the mass of a carbon-12 atom which is given the relative atomic mass of 12.000.
Some periodic tables show the mass number of the most common isotope. Some show the relative atomic mass. Although it is wrong to say that they are the same thing (because they are not!) the difference will not be exploited at GCSE.
Relative Molecular Mass - This is the sum of all the relative atomic masses of all of the atoms in a compound e.g.
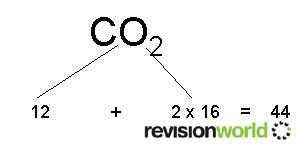
Reacting Masses
By using the relative molecular masses in grams we can deduce what masses of reactants to use and what mass of products will be formed.
The Law of Conservation of Mass says that the total mass of the strating materials is the same as the total mass of the products.
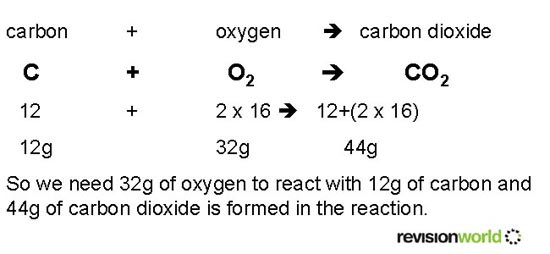
The Mole
One mole of atoms or molecule of any substance will have a mass in grams equal to the relative atomic mass or relative molecular mass for that substance.
The atomic mass of carbon is 12, therefore one mole of carbon weighs 12g
The relative molecular mass of oxygen (O2) is (2 x 16) 32, therefore one mole of oxygen weighs 32g.
One mole of any substance contains 6.02 x 1023 atoms or molecule.
Remember the mole is just a number!
Titrations
Titrations This is also known as volumetric analysis.
We try to find the volumes of acid and alkali that just cancel each other out. At first glance it might seem that 25ml of acid will always cancel 25ml of alkali but this is not the case.
Suppose that the alkali is not the same concentration as the acid. A few drops of a very concentrated alkali may neutralise a bucketful of a dilute acid.
Some people like to follow simple formulae while others like to work out what is really happening.
For the simple formula people...... concentration of acid x volume of acid = concentration of alkali x volume of alkali (This assumes that the reaction is a 1:1 type and that the units are consistent)
Titrations (with thinking)
- In an experiment, 20.6 cm3 of a 0.87 mol/dm3 solution of an acid just react with 29.7 cm3 of an unknown alkali.
- We know that the acid and alkali react in a 1:1 reaction.
- At the end point the number of moles of H+ and the number of moles of OH- is the same.
- We can calculate the number of moles of H+ as follows:
- For 20.6 cm3 of a 0.87 mol/dm3 solution of an acid.
The following statement is always true…….
- 1 dm3 of a 1 mol/dm3 solution of acid must contain 1 mole of acid, so
- 1 dm3 of a 0.87 mol/dm3 solution of acid must contain 0.87 moles of acid, so
- 1 cm3 of a 0.87 mol/dm3 solution of acid must contain 0.87 x 1/1000 moles of acid so,
- 20.6 cm3 of a 0.87 mol/dm3 solution of acid contains 20.6 x 0.87/1000 moles of acid.
- So, 0.0179 moles of acid were used.
At the end point, we know that the moles of acid and alkali are the same.
So, there must have been 0.0179 moles of alkali.
These moles of alkali were contained in the 29.7cm3. Use the same idea as above:
- 29.7 cm3 of alkali contain 0.0179 moles of alkali, so
- 1 cm3 of alkali must contain 0.0179/29.7 moles of alkali, so
- 1 dm3 of alkali must contain 0.0179 x 1000/29.7 moles of alkali.
- So, the concentration of the alkali is 0.60 .
