Energy Transfer in Reactions
This section explains Energy Transfer in Reactions.
Image
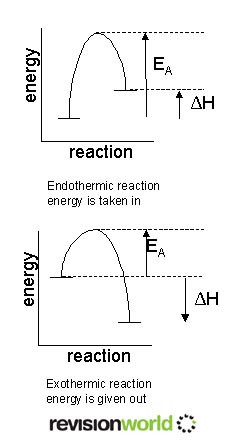
Breaking Bonds
- Chemical reactions involve the making and breaking of bonds.
- Energy must be taken in so that bonds can be broken.
- Energy is given out when new bond are made.
- EA is the activation energy this is the energy required to break the bond of the reactants.
- AH is the energy (enthalpy) change of the reaction if it is negative energy is given out if it is positive energy is taken in.
- In an exothermic reaction more energy is released making new bonds than is taken in breaking bonds.
- In an endothermic reaction less energy is released making new bonds than is taken in breaking bonds.
The energy change of a reaction can be calculated using the following formula:
AH = Energy used in breaking bonds- Energy given out in making bonds
Remember for a particular bond the energy for breaking and making it is the same.
This video explains more about breaking bonds
