Radioactive Decay, Nuclear Radiation and Nuclear Equations
This section explores radioactive decay, nuclear radiation and nuclear equations covering, radioactive decay, nuclear radiation, a comparison table of nuclear radiation types, nuclear equations and alpha, beta and gamma decay.
Radioactive Decay
Radioactive decay is a random process by which an unstable atomic nucleus loses energy by emitting radiation. This decay continues until the atom becomes stable, often producing a different element or isotope.
Activity and Count Rate
- Activity is a measure of the number of decays per second in a sample of radioactive material. It is measured in becquerels (Bq), where 1 Bq is equivalent to 1 decay per second.
- Count rate refers to the number of particles detected by a Geiger-Müller tube or other detector per second. It is often measured in counts per minute (cpm) or counts per second (cps).
The activity of a sample decreases over time as fewer unstable nuclei remain to decay, so the count rate will decrease as well. This process follows an exponential decay pattern.
Nuclear Radiation
There are three main types of nuclear radiation emitted during radioactive decay:
- Alpha Particles
- Beta Particles
- Gamma Rays
These types of radiation differ in their properties, such as their ability to penetrate materials, their ionising power, and how they are absorbed.
Comparison Table of Nuclear Radiation Types
| Particles | Description | Penetration in Air | Absorbed By | Ionising Power |
|---|---|---|---|---|
| Alpha Particles | Consist of 2 protons and 2 neutrons (helium nucleus). | Very low: travels only a few cm in air. | Stopped by paper, skin, or a few centimetres of air. | Very high: can ionise atoms easily but has low penetrating power. |
| Beta Particles | High-energy electrons emitted from the nucleus of an atom. | Moderate: travels several metres in air. | Stopped by a few millimetres of aluminium or plastic. | Moderate: ionises atoms but is less ionising than alpha particles. |
| Gamma Rays | Electromagnetic radiation with very high frequency and energy. | Very high: travels hundreds of metres in air. | Stopped by thick layers of lead or concrete. | Low: very weak ionising power, but highly penetrating. |
Nuclear Equations
Nuclear equations show the process of radioactive decay. In these equations, the mass number (A) and atomic number (Z) are conserved.
When an unstable nucleus emits radiation, a new element may be formed. Here’s how each type of radiation is represented in nuclear equations:
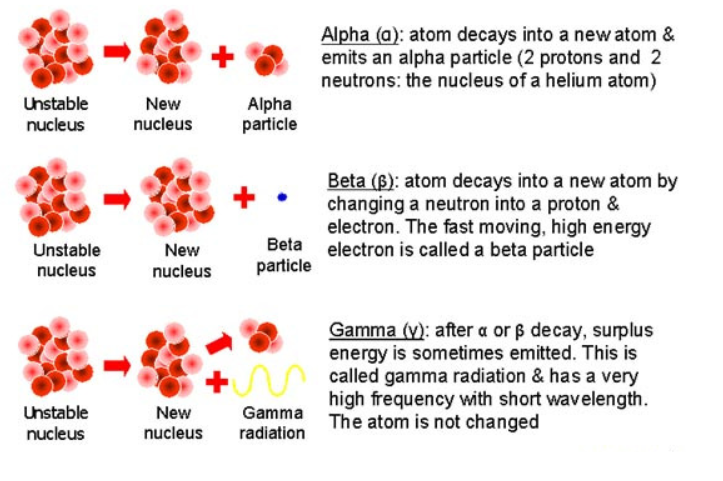
Alpha Decay
When an atom emits an alpha particle, it loses 2 protons and 2 neutrons. The mass number decreases by 4, and the atomic number decreases by 2.
Example: $\text{Uranium-238} \rightarrow \text{Thorium-234} + \text{Alpha Particle} _{92}^{238}\text{U} \rightarrow _{90}^{234}\text{Th} + _{2}^{4}\alpha$
Beta Decay
When a nucleus emits a beta particle (an electron), a neutron in the nucleus is converted into a proton. The atomic number increases by 1, but the mass number remains unchanged.
Example: $\text{Carbon-14} \rightarrow \text{Nitrogen-14} + \text{Beta Particle} _{6}^{14}\text{C} \rightarrow _{7}^{14}\text{N} + _{-1}^{0}\beta$
Gamma Decay
Gamma radiation involves the release of electromagnetic waves (gamma rays) from a nucleus. Gamma decay usually happens after an alpha or beta decay, when the nucleus has excess energy. Gamma decay does not change the atomic number or mass number of the atom.
Example: $\text{Cobalt-60} \rightarrow \text{Cobalt-60} + \text{Gamma Ray} _{27}^{60}\text{Co} \rightarrow _{27}^{60}\text{Co} + \gamma$
Key Points to Remember:
- Radioactive decay is a random process where an unstable nucleus loses energy by emitting radiation.
- Activity is the number of decays per second and is measured in becquerels (Bq).
- Count rate is the number of particles detected by a radiation detector.
- There are three main types of radiation: alpha particles, beta particles, and gamma rays, each with different properties.
- Nuclear equations show how atoms change during radioactive decay and conserve mass and atomic numbers.
Understanding radioactive decay and nuclear radiation is essential in fields such as nuclear medicine, environmental science, and nuclear energy.
