Changes of State and the Particle Model
This section explains charges of state and the particle model covering, the density of material equation, ice, water and steam, internal energy, changes of heat and specific latent heat and the energy required to cause a change of state equation.
The Particle Model
The Particle Model of matter explains how the properties of solids, liquids, and gases are related to the arrangement and movement of particles (atoms or molecules).
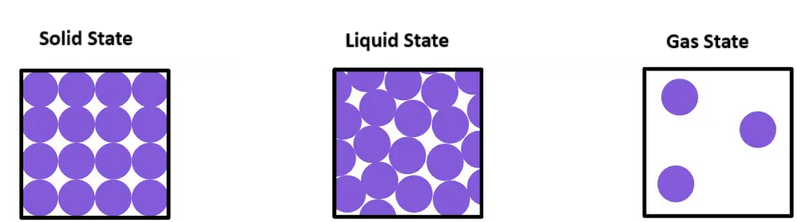
- Solids: In solids, particles are closely packed together in a regular arrangement. They vibrate in place but do not move from their fixed positions. This gives solids a fixed shape and volume.
- Liquids: In liquids, particles are still close together but can move past one another. This allows liquids to flow and take the shape of their container while maintaining a fixed volume.
- Gases: In gases, particles are far apart and move freely at high speeds. This means gases have neither a fixed shape nor a fixed volume, and they expand to fill the container they are in.
The state of matter is determined by the amount of energy the particles have and the forces acting between them.
The Density of Material Equation
Density is a measure of how much mass is contained in a given volume of a material. It can be calculated using the following equation:
Density (ρ) = Mass (m) / Volume (V)
Where:
- ρ is the density in kilograms per cubic metre (kg/m³),
- m is the mass in kilograms (kg),
- V is the volume in cubic metres (m³).
Example:
If you have a block of material with a mass of 200 kg and a volume of 0.5 m³, you can calculate its density:
$$ρ = \frac{m}{V} = \frac{200 \, \text{kg}}{0.5 \, \text{m}^3} = 400 \, \text{kg/m}^3$$
So, the density of the material is 400 kg/m³.
Ice, Water, and Steam
When a substance changes from one state to another, its particles undergo changes in arrangement and energy.
- Ice (Solid): In the solid state (ice), water molecules are arranged in a regular pattern and vibrate in place.
- Water (Liquid): As ice melts and becomes liquid water, the particles move more freely while staying close together, allowing the liquid to flow and take the shape of its container.
- Steam (Gas): When water is heated to its boiling point, the particles gain enough energy to break free from the liquid and move independently as a gas (steam), which expands to fill its container.
Each state has distinct properties due to the arrangement and movement of particles, which change when energy is added or removed.
Internal Energy
Internal energy refers to the total energy stored within a substance due to the movement and arrangement of its particles. This energy is made up of:
- Kinetic energy: The energy of particles moving.
- Potential energy: The energy stored in the arrangement of particles, especially when they are close together or far apart.
When a substance is heated, its particles move faster, increasing their kinetic energy and, therefore, the internal energy of the substance. This leads to changes in temperature or a change of state.
Changes of Heat and Specific Latent Heat
When a substance changes state (for example, from solid to liquid or liquid to gas), latent heat is involved. Latent heat is the energy required to change the state of a substance without changing its temperature.
Specific latent heat is the amount of energy needed to change the state of 1 kg of a substance without a change in temperature.
The equation for specific latent heat (L) is:
$$Q = m \times L$$
Where:
- Q is the heat energy (in joules, J),
- m is the mass of the substance (in kilograms, kg),
- L is the specific latent heat (in joules per kilogram, J/kg).
The Energy Required to Cause a Change of State Equation
To change the state of a substance, energy is required. This energy is known as latent heat and depends on the substance’s mass and its specific latent heat.
Equation:
$$Q = m \times L$$
Where:
- Q is the energy required to change the state (in joules, J),
- m is the mass of the substance (in kilograms, kg),
- L is the specific latent heat (in joules per kilogram, J/kg).
Example:
Let’s say we want to melt 2 kg of ice, and the specific latent heat of fusion for ice is 334,000 J/kg. To find the energy required to melt the ice, we can use the equation:
$$Q = m \times L$$
$$Q = 2 \, \text{kg} \times 334,000 \, \text{J/kg} = 668,000 \, \text{J}$$
So, the energy required to melt 2 kg of ice is 668,000 joules.
Summary
- The Particle Model explains how the arrangement and movement of particles determine the properties of solids, liquids, and gases.
- Density can be calculated using the formula Density (ρ) = Mass (m) / Volume (V).
- Ice, water, and steam represent different states of matter, with particles arranged differently and possessing different amounts of energy.
- Internal energy is the total energy within a substance due to particle movement and arrangement.
- Specific latent heat is the energy required to change the state of 1 kg of a substance without changing its temperature.
- The energy required to cause a change of state can be calculated using the formula Q = m × L.
Understanding these concepts is crucial for explaining how energy is transferred during changes of state and how substances behave under different temperature and pressure conditions.
