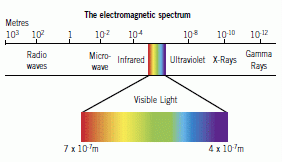
The Electromagnetic Spectrum
The Electromagnetic Spectrum
The spectrum of electromagnetic waves is continuous from the longest wavelengths (radio waves) through to the shortest wavelengths (gamma rays).
All electromagnetic waves are transverse waves that can travel through a vacuum. They all travel through empty space at a speed of 300 000 000 m/s.
Radio waves have the longest wavelength, lowest frequency and lowest energy. Gamma rays have the shortest wavelength, highest frequency and highest energy. The wavelengths are related to the frequencies using the wave equation.
This video provides an introduction to the Electromagnetic Spectrum
This video explains what happens when you mix colours from the electromagnetic spectrum
Radiation
Radiation is transmitted from the source to the detector. Some materials absorb some types of electromagnetic radiation. The further the radiation travels through an absorbent material the lower its intensity will be when it reaches the end of its journey. More of the radiation energy is absorbed by the material and it heats up.
On some journeys the radiation is reflected at a boundary between two different materials.
When the energy of the radiation is absorbed by the detector it may:
- Have a heating effect.
- Produce small electric currents in aerials (if microwaves or radio waves).
- Make chemical reactions more likely, for example light causes photosynthesis in plants.
- Ionise atoms (if ultraviolet, X-ray or gamma).
Energy and Intensity
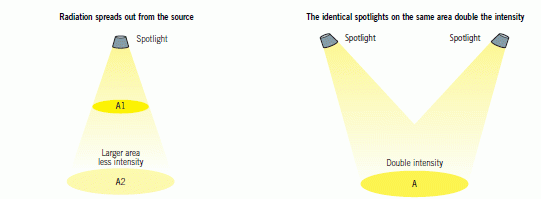
When electromagnetic radiation strikes a surface, the intensity of the radiation is the amount of energy arriving at a square metre of the surface each second.
Radiation is emitted from a source and travels towards a destination. On this journey the radiation spreads out, so the further away a detector is from the source the less energy is detected. The intensity of the radiation can be increased by moving closer to the source.
Intensity can also be increased by increasing the radiation from the source.
The energy absorbed by the surface or detector can be calculated by:
Energy (J) = intensity (W/m2) × time (s)
Electromagnetic radiation sometimes behaves as waves, and sometimes as packets of energy called photons. A gamma ray photon has the most energy. The energy of the photons increases with the frequency of the radiation.

