Reaction Rates
The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. Its units are mol dm-3s-1/.
Factors that influence rate of reaction:
-
the concentration of the reactants
-
the surface area of solid reactants
-
a temperature change
-
the presence of a catalyst.
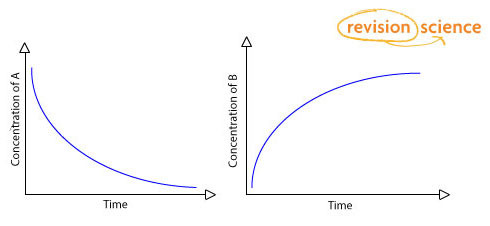
For a reaction of A → B the reaction rate is (change of concentration)/time.
It can be measured as the rate of:
decrease in concentration of A
increase in concentration of B
If plotted on a graph, the concentration of A would decrease over time and the concentration of B would increase.
The gradient of the graph indicates the rate of reaction – the steeper the gradient the faster the rate of reaction.
The reaction will be greatest when the concentration of A is greatest and will slow down as the concentration of A decreases.
When the reaction is complete the graphs level off and the gradient is equal to zero.
Effect of Concentration on Reaction Rates
Reaction Rate increases with concentration as there are more particles present per volume and more collisions take place – with more collisions exceeding the activation energy per second.
Provided the temperature stays the same, Boltzmann Distribution curves for different concentrations will have the same shape.
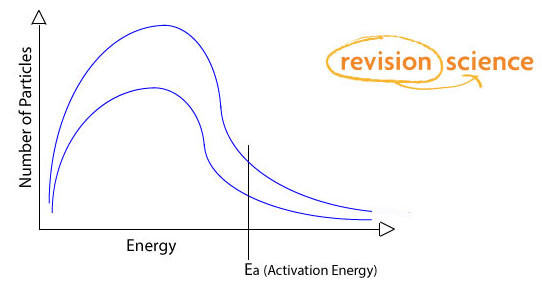
For reactions involving gases, increasing the pressure also increases the concentration, resulting in an increased reaction rate.
Effect of Surface area on Reaction Rates
For a reaction involving a solid, the greater the surface area the greater the Reaction Rate. This is because more of the solid is exposed to be reacted with and therefore more collisions occur per second.
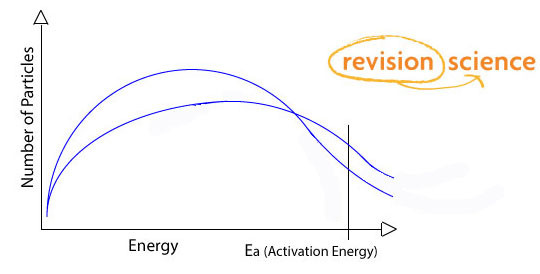
Effect of Temperature on Reaction Rates
The average kinetic energy of the particles is proportional with temperature - as temperature increases, so does the kinetic energy of gas particles.
With this increased energy molecules move faster and therefore there are more collisions each second.
Also a greater proportion of molecules exceed the activation energy.
For a higher temperature the Boltzmann distribution curve is displaced to the right with a lower peak (provided all other factors remain constant).